Acquisition of APIRx
For personal use only
Canchew and Chewell patented MCGTs
for Over-the-Counter ('OTC') and Prescription markets
01.
MCGTs, using APIRX patented formulation
technology, with potential to develop as OTC
products in Australia and other jurisdictions
(U.S., EU, UK, et cetera).
DE LO
02.
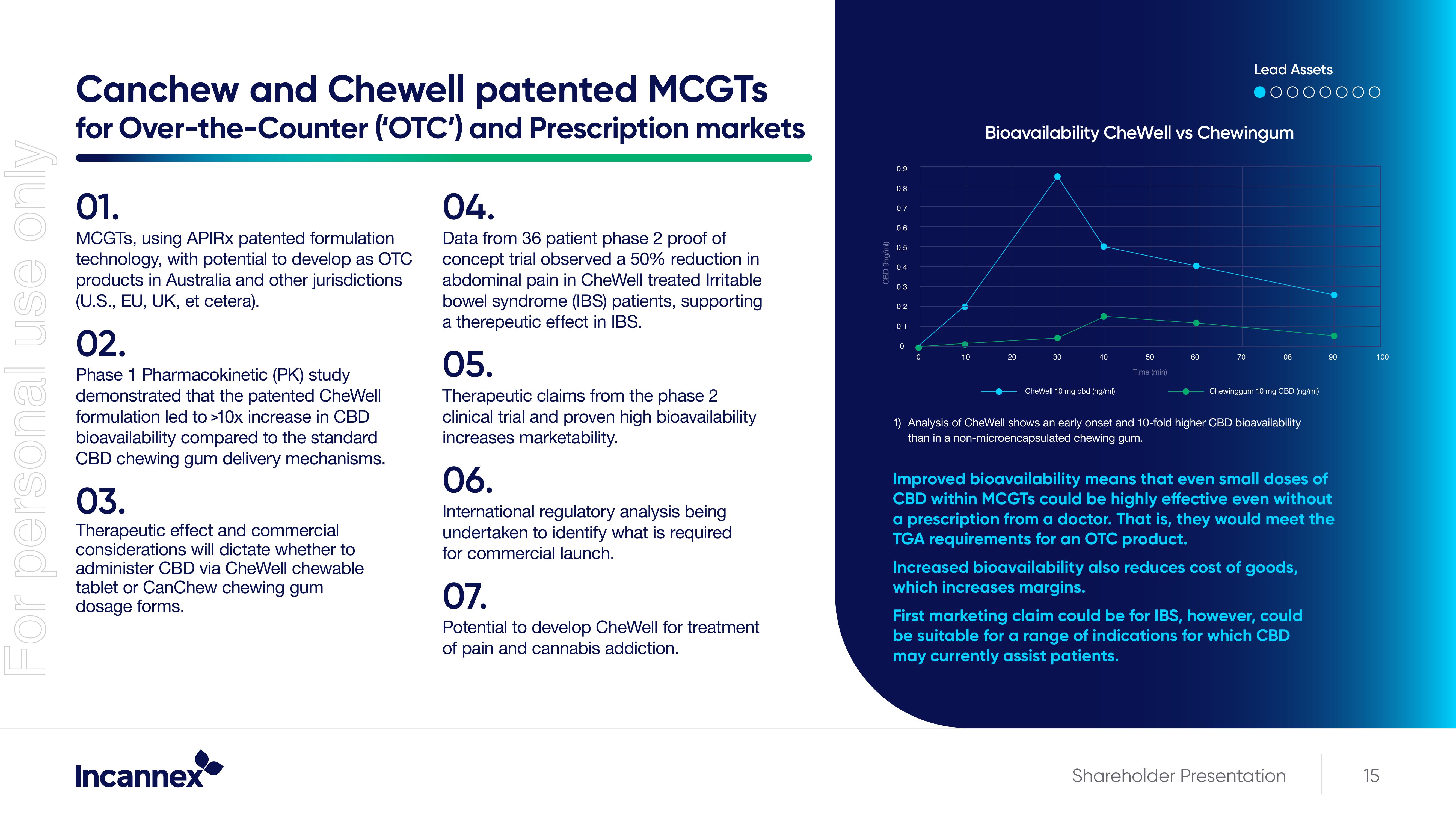
Phase 1 Pharmacokinetic (PK) study
demonstrated that the patented CheWell
formulation led to >10x increase in CBD
bioavailability compared to the standard
CBD chewing gum delivery mechanisms.
03.
Therapeutic effect and commercial
considerations will dictate whether to
administer CBD via CheWell chewable
tablet or CanChew chewing gum
dosage forms.
Incannex
04.
Data from 36 patient phase 2 proof of
concept trial observed a 50% reduction in
abdominal pain in CheWell treated Irritable
bowel syndrome (IBS) patients, supporting
a therepeutic effect in IBS.
05.
Therapeutic claims from the phase 2
clinical trial and proven high bioavailability
increases marketability.
06.
International regulatory analysis being
undertaken to identify what is required
for commercial launch.
07.
Potential to develop CheWell for treatment
of pain and cannabis addiction.
CBD 9ng/ml)
0,9
0.8
0,7
0,6
0.5
0,4
0,3
0,2
0,1
0
0
10
Bioavailability CheWell vs Chewingum
20
30
40
CheWell 10 mg cbd (ng/ml)
50
Time (min)
60
Lead Assets
70
08
Chewinggum 10 mg CBD (ng/ml)
1) Analysis of CheWell shows an early onset and 10-fold higher CBD bioavailability
than a non-microencapsulated chewing gum.
Improved bioavailability means that even small doses of
CBD within MCGTs could be highly effective even without
a prescription from a doctor. That is, they would meet the
TGA requirements for an OTC product.
Increased bioavailability also reduces cost of goods,
which increases margins.
First marketing claim could be for IBS, however, could
be suitable for a range of indications for which CBD
may currently assist patients.
90
Shareholder Presentation
100
15View entire presentation