Equillium Results Presentation Deck
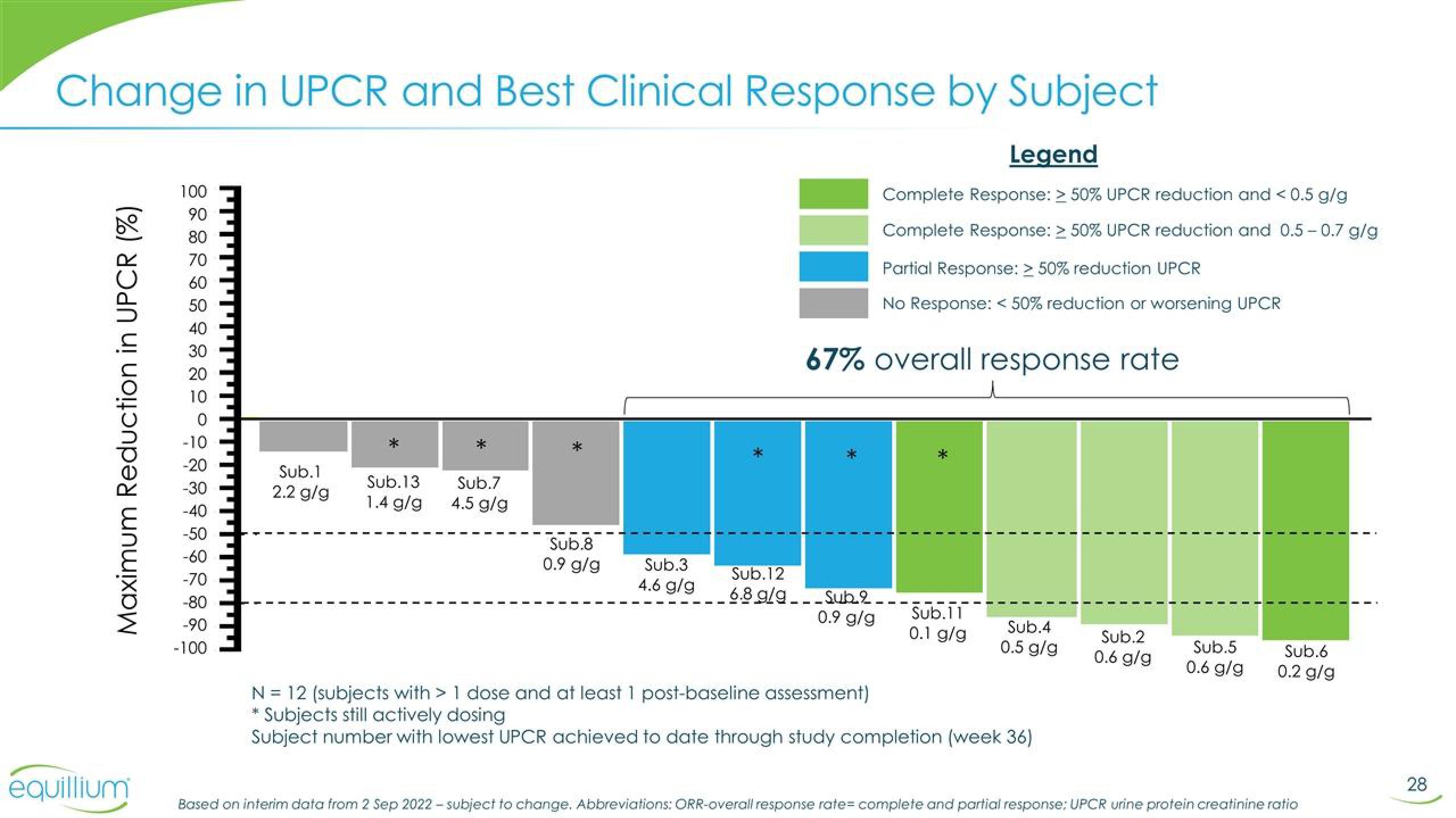
Change in UPCR and Best Clinical Response by Subject
Legend
Complete Response: > 50% UPCR reduction and < 0.5 g/g
Complete Response: > 50% UPCR reduction and 0.5-0.7 g/g
Partial Response: > 50% reduction UPCR
No Response: < 50% reduction or worsening UPCR
Maximum Reduction in UPCR (%)
equillium
100
90
80
70
60
50
40
30
20
10
0
-10
-20
-30
-40
-50
-60
-70
-80
-90
-100
Sub.1
2.2 g/g
Sub.13 Sub.7
1.4 g/g 4.5 g/g
Sub.8
0.9 g/g
Sub.3
4.6 g/g
*
Sub.12
6.8 g/g
67% overall response rate
Sub.2.
0.9 g/g
*
Sub.11
0.1 g/g
Sub.4
0.5 g/g
N = 12 (subjects with > 1 dose and at least 1 post-baseline assessment)
*
Subjects still actively dosing
Subject number with lowest UPCR achieved to date through study completion (week 36)
Sub.2
0.6 g/g
Sub.5
0.6 g/g
Sub.6
0.2 g/g
Based on interim data from 2 Sep 2022-subject to change. Abbreviations: ORR-overall response rate= complete and partial response: UPCR urine protein creatinine ratio
28View entire presentation