Connecting Innovation to Purpose
●
●
●
8
Padcev® is associated with skin toxicities and peripheral neuropathy
PADCEV.Ⓡ
enfortumab vedotin-ejfv
Injection for IV infusion 20 mg & 30 mg vials
A Black Box warning 1
WARNING: SERIOUS SKIN REACTIONS
PADCEV can cause severe and fatal cutaneous adverse reactions including Stevens-Johnson syndrome
(SJS) and Toxic Epidermal Necrolysis (TEN), which occurred predominantly during the first cycle of
treatment, but may occur later.
Closely monitor patients for skin reactions.
Immediately withhold PADCEV and consider referral for specialized care for suspected SJS or TEN or
severe skin reactions.
Permanently discontinue PADCEV in patients with confirmed SJS or TEN; or Grade 4 or recurrent
Grade 3 skin reactions (see Dosage and Administration (2.2), Warnings and Precautions (5.1) and Adverse
Reactions (6.1)].
Greater than 25% of PADCEV® discontinuations are
linked to peripheral neuropathy2
PADCEVⓇ + Keytruda® patients who experienced
neuropathy:
13% complete resolution
87% patients had residual neuropathy
(45% had Grade ≥2]¹
●
Source(s): 1. PADCEVⓇ Prescribing Information Dec 2023. 2.. Rosenberg et al., 2020
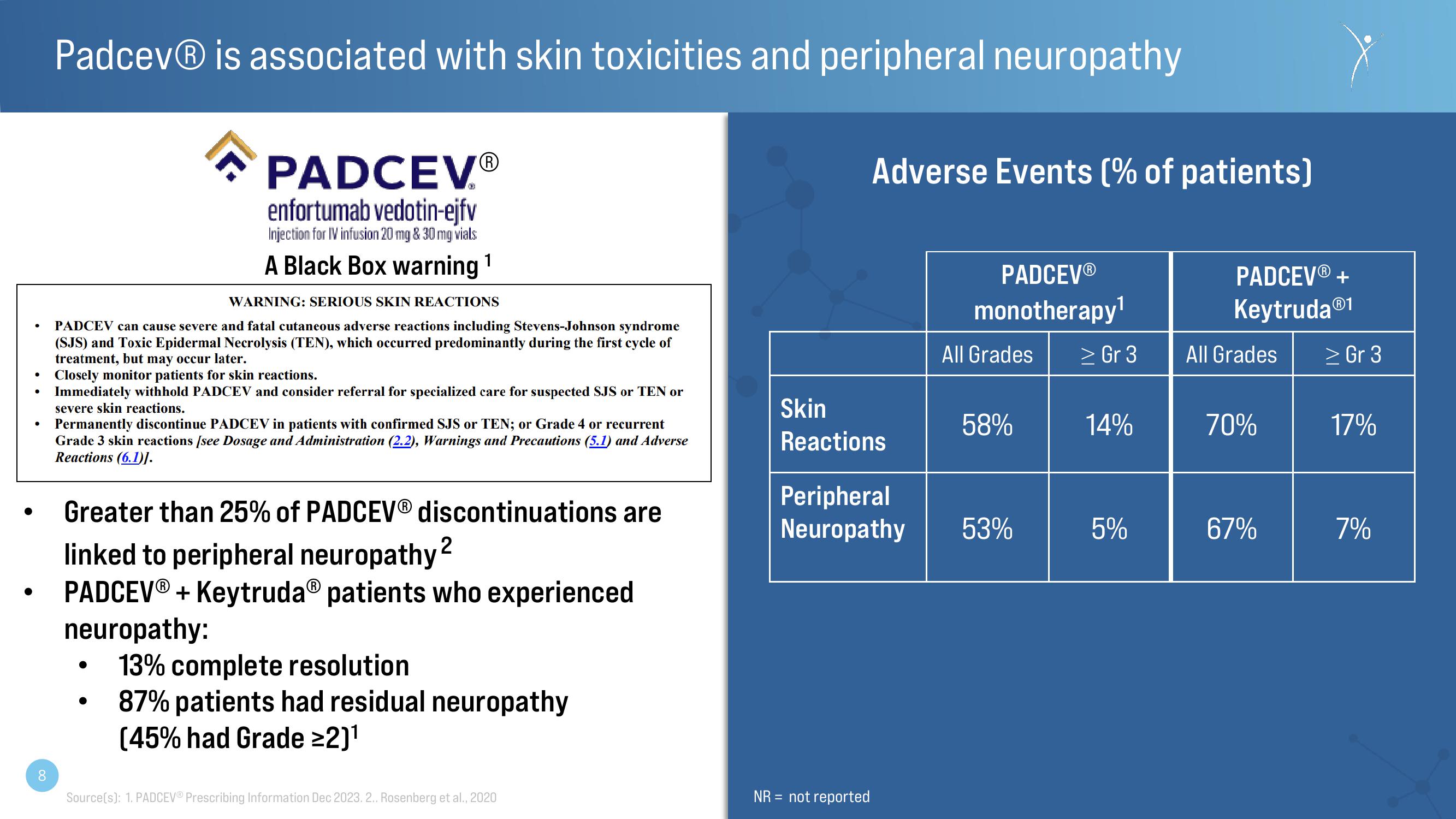
Adverse Events (% of patients)
Skin
Reactions
NR = not reported
PADCEVⓇ
monotherapy¹
All Grades
58%
Peripheral
Neuropathy 53%
> Gr 3
14%
5%
PADCEVⓇ+
Keytruda Ⓡ1
All Grades
70%
67%
> Gr 3
17%
7%View entire presentation