BioNTech Results Presentation Deck
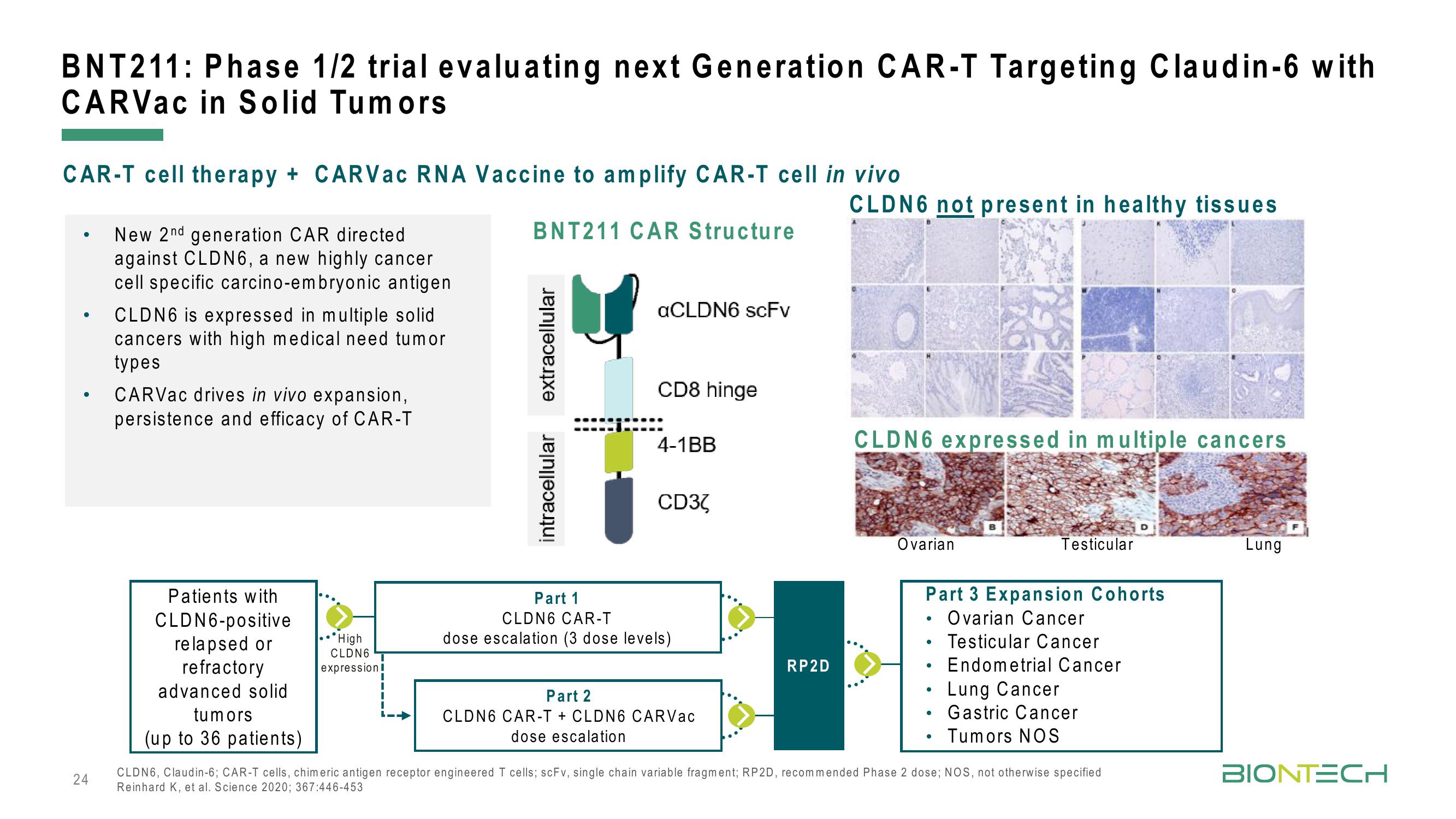
BNT211: Phase 1/2 trial evaluating next Generation CAR-T Targeting Claudin-6 with
CARVac in Solid Tumors
CAR-T cell therapy + CARVac RNA Vaccine to amplify CAR-T cell in vivo
BNT211 CAR Structure
●
●
●
24
New 2nd generation CAR directed
against CLDN6, a new highly cancer
cell specific carcino-embryonic antigen
CLDN6 is expressed in multiple solid
cancers with high medical need tumor
types
CARVac drives in vivo expansion,
persistence and efficacy of CAR-T
Patients with
CLDN6-positive
relapsed or
refractory
advanced solid
tumors
(up to 36 patients)
.. High
CLDN6
expression
extracellular
intracellular
aCLDN6 scFv
CD8 hinge
4-1BB
CD32
Part 1
CLDN6 CAR-T
dose escalation (3 dose levels)
Part 2
CLDN6 CAR-T + CLDN6 CARVac
dose escalation
RP2D
CLDN6 not present in healthy tissues
0
CLDN6 expressed in multiple cancers
Ovarian
Part 3 Expansion Cohorts
Ovarian Cancer
Testicular Cancer
Endometrial Cancer
●
●
●
●
●
Testicular
●
Lung Cancer
Gastric Cancer
Tumors NOS
CLDN6, Claudin-6; CAR-T cells, chimeric antigen receptor engineered T cells; scFv, single chain variable fragment; RP2D, recommended Phase 2 dose; NOS, not otherwise specified
Reinhard K, et al. Science 2020; 367:446-453
Lung
BIONTECHView entire presentation