OmniAb SPAC Presentation Deck
ANTIBODIES AND INDUSTRY DEMAND
HIGHER SUCCESS RATES FOR ANTIBODY MEDICINES DRIVE OUR INDUSTRY'S
NEED FOR DISCOVERY TECHNOLOGY
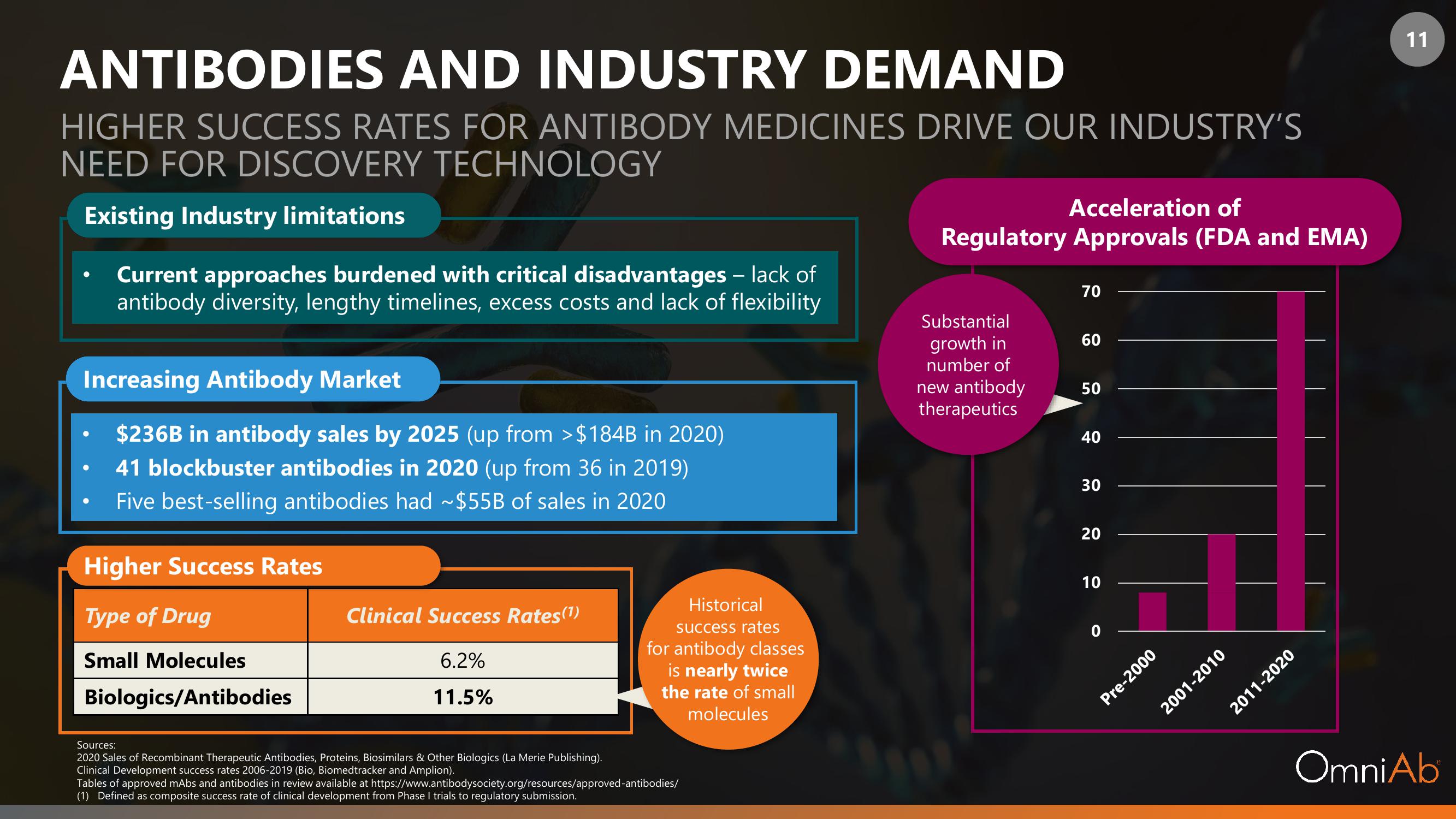
Existing Industry limitations
Current approaches burdened with critical disadvantages - lack of
antibody diversity, lengthy timelines, excess costs and lack of flexibility
Increasing Antibody Market
$236B in antibody sales by 2025 (up from >$184B in 2020)
41 blockbuster antibodies in 2020 (up from 36 in 2019)
Five best-selling antibodies had ~$55B of sales in 2020
●
Higher Success Rates
Type of Drug
Small Molecules
Biologics/Antibodies
Clinical Success Rates (¹)
6.2%
11.5%
Sources:
2020 Sales of Recombinant Therapeutic Antibodies, Proteins, Biosimilars & Other Biologics (La Merie Publishing).
Clinical Development success rates 2006-2019 (Bio, Biomed tracker and Amplion).
Historical
success rates
for antibody classes
is nearly twice
the rate of small
molecules
Tables of approved mAbs and antibodies in review available at https://www.antibodysociety.org/resources/approved-antibodies/
(1) Defined as composite success rate of clinical development from Phase I trials to regulatory submission.
Acceleration of
Regulatory Approvals (FDA and EMA)
Substantial
growth in
number of
new antibody
therapeutics
70
60
50
40
30
20
10
Pre-2000
2011-2020
2001-2010
11
OmniAbView entire presentation