Calliditas Therapeutics IPO Presentation Deck
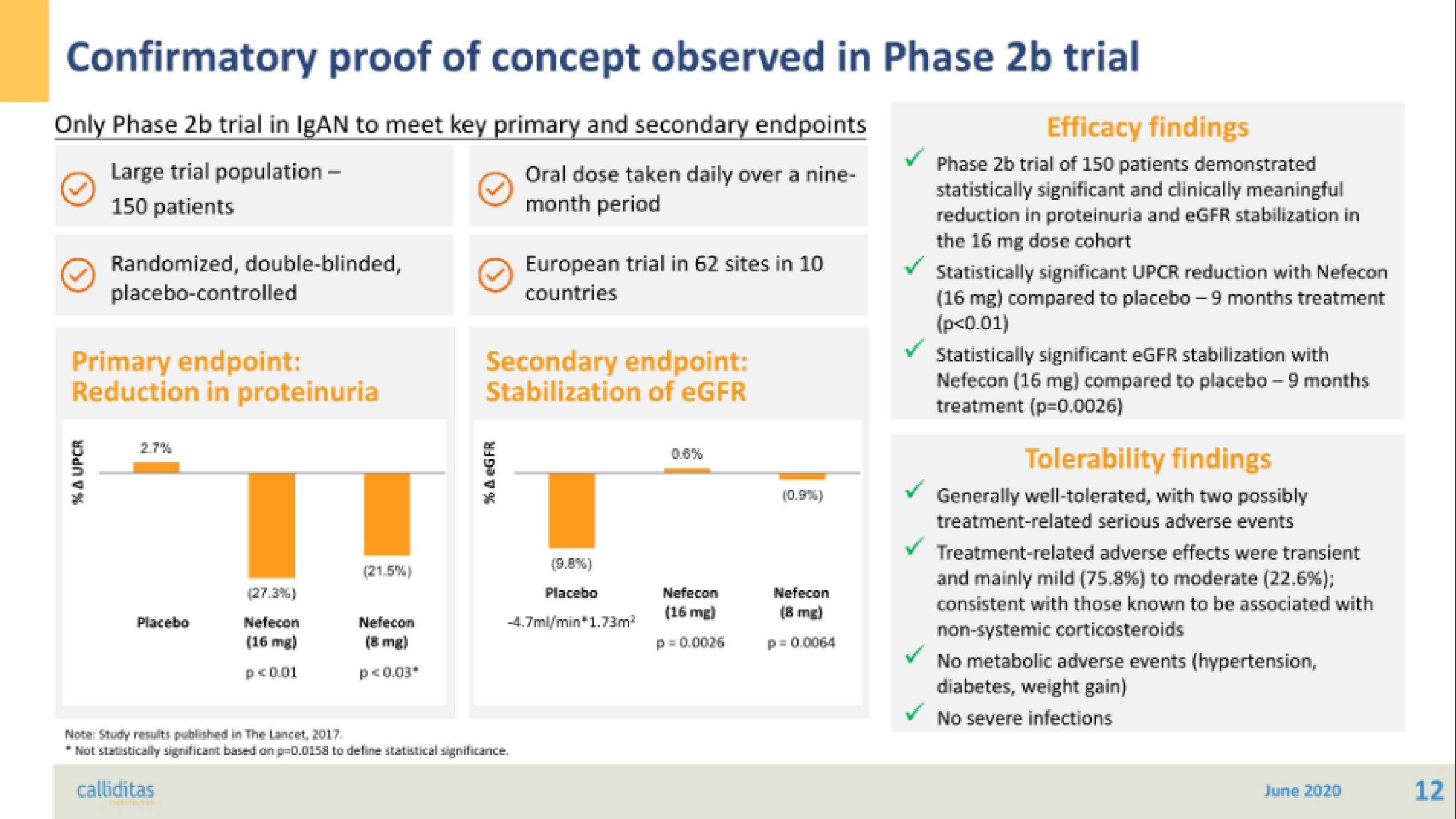
Confirmatory proof of concept observed in Phase 2b trial
Only Phase 2b trial in IgAN to meet key primary and secondary endpoints
Large trial population -
Oral dose taken daily over a nine-
month period
150 patients
Randomized, double-blinded,
placebo-controlled
Primary endpoint:
Reduction in proteinuria
% & UPCR
Placebo
(27.3%)
Nefecon
(16 mg)
p < 0.01
calliditas
B
(21.5%)
Nefecon
p<0.03*
Secondary endpoint:
Stabilization of eGFR
% A eGFR
European trial in 62 sites in 10
countries
Note: Study results published in The Lancet, 2017.
* Not statistically significant based on p-0.0158 to define statistical significance.
Placebo
-4.7ml/min*1.73m²
Nefecon
(16 mg)
p = 0.0026
Nefecon
(8 mg)
p = 0.0064
L
wwww
Efficacy findings
Phase 2b trial of 150 patients demonstrated
statistically significant and clinically meaningful
reduction in proteinuria and eGFR stabilization in
the 16 mg dose cohort
Statistically significant UPCR reduction with Nefecon
(16 mg) compared to placebo - 9 months treatment
(p<0.01)
Statistically significant eGFR stabilization with
Nefecon (16 mg) compared to placebo - 9 months
treatment (p=0.0026)
Tolerability findings
Generally well-tolerated, with two possibly
treatment-related serious adverse events
Treatment-related adverse effects were transient
and mainly mild (75.8%) to moderate (22.6%);
consistent with those known to be associated with
non-systemic corticosteroids
No metabolic adverse events (hypertension,
diabetes, weight gain)
No severe infections
June 2020
12View entire presentation