AstraZeneca Results Presentation Deck
16
CEO Opening Remarks
Financial Results
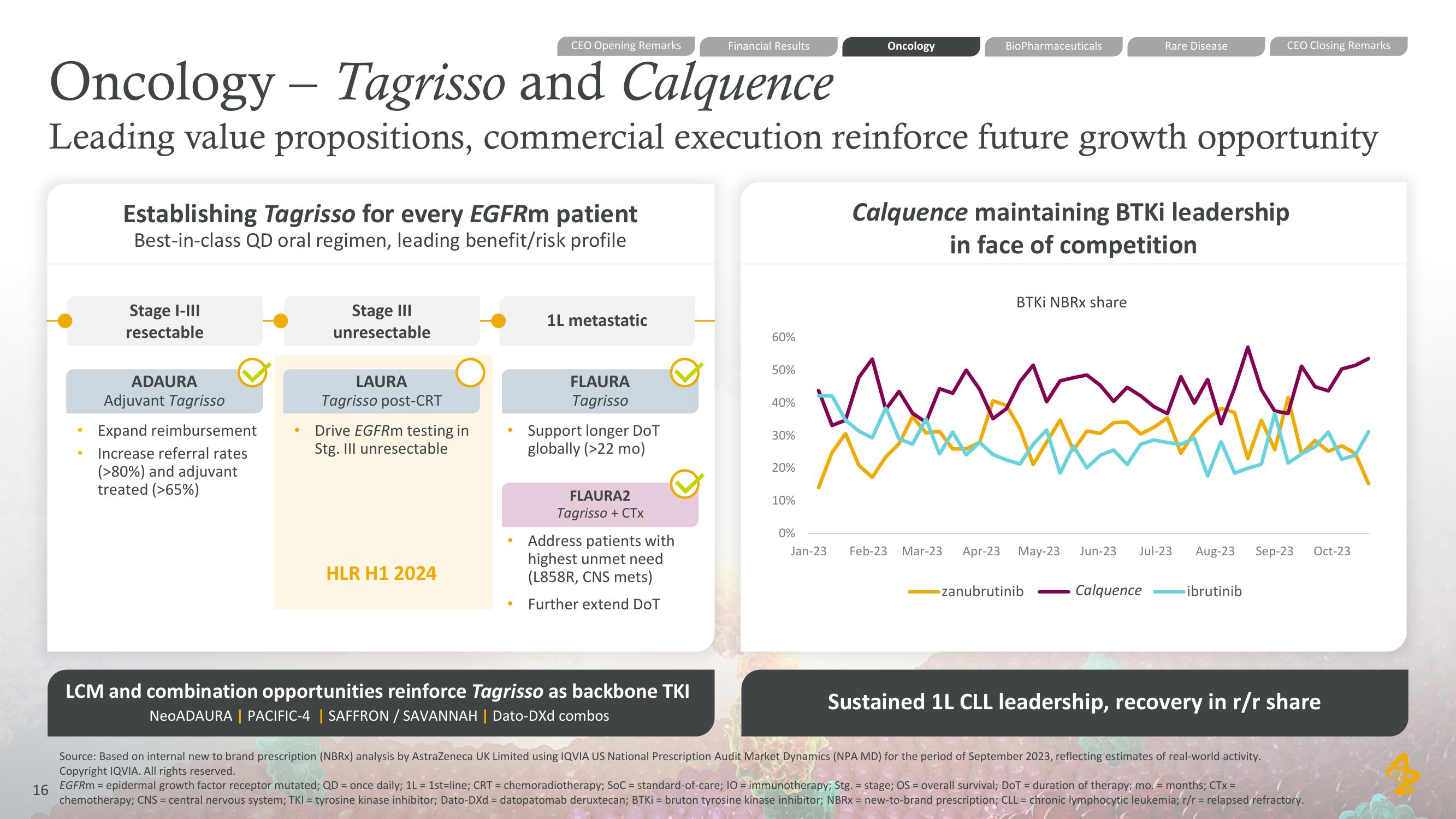
Oncology - Tagrisso and Calquence
Leading value propositions, commercial execution reinforce future growth opportunity
●
●
Establishing Tagrisso for every EGFRm patient
Best-in-class QD oral regimen, leading benefit/risk profile
Stage I-III
resectable
ADAURA
Adjuvant Tagrisso
Expand reimbursement
Increase referral rates
(>80%) and adjuvant
treated (>65%)
Stage III
unresectable
LAURA
Tagrisso post-CRT
Drive EGFRm testing in
Stg. Ill unresectable
HLR H1 2024
1L metastatic
FLAURA
Tagrisso
Support longer DoT
globally (>22 mo)
FLAURA2
Tagrisso + CTX
Address patients with
highest unmet need
(L858R, CNS mets)
Further extend DoT
LCM and combination opportunities reinforce Tagrisso as backbone TKI
NeoADAURA | PACIFIC-4 | SAFFRON / SAVANNAH | Dato-DXd combos
60%
50%
40%
30%
20%
10%
Oncology
BioPharmaceuticals
BTKI NBRx share
Calquence maintaining BTKi leadership
in face of competition
m
say
Rare Disease
zanubrutinib
Calquence
CEO Closing Remarks
0%
Jan-23 Feb-23 Mar-23 Apr-23 May-23 Jun-23 Jul-23 Aug-23 Sep-23 Oct-23
ww
Whax
ibrutinib
Sustained 1L CLL leadership, recovery in r/r share
Source: Based on internal new to brand prescription (NBRx) analysis by AstraZeneca UK Limited using IQVIA US National Prescription Audit Market Dynamics (NPA MD) for the period of September 2023, reflecting estimates of real-world activity.
Copyright IQVIA. All rights reserved.
EGFRM= epidermal growth factor receptor mutated; QD = once daily; 1L = 1st-line; CRT = chemoradiotherapy; SoC = standard-of-care; 10 = immunotherapy; Stg. = stage; OS = overall survival; DoT= duration of therapy; mo. = months; CTX =
chemotherapy; CNS = central nervous system; TKI = tyrosine kinase inhibitor; Dato-DXd = datopatomab deruxtecan; BTKI = bruton tyrosine kinase inhibitor; NBRX = new-to-brand prescription; CLL = chronic lymphocytic leukemia; r/r = relapsed refractory.View entire presentation