Ocuphire Pharma Results Presentation Deck
RM
Percent of Subjects (%)
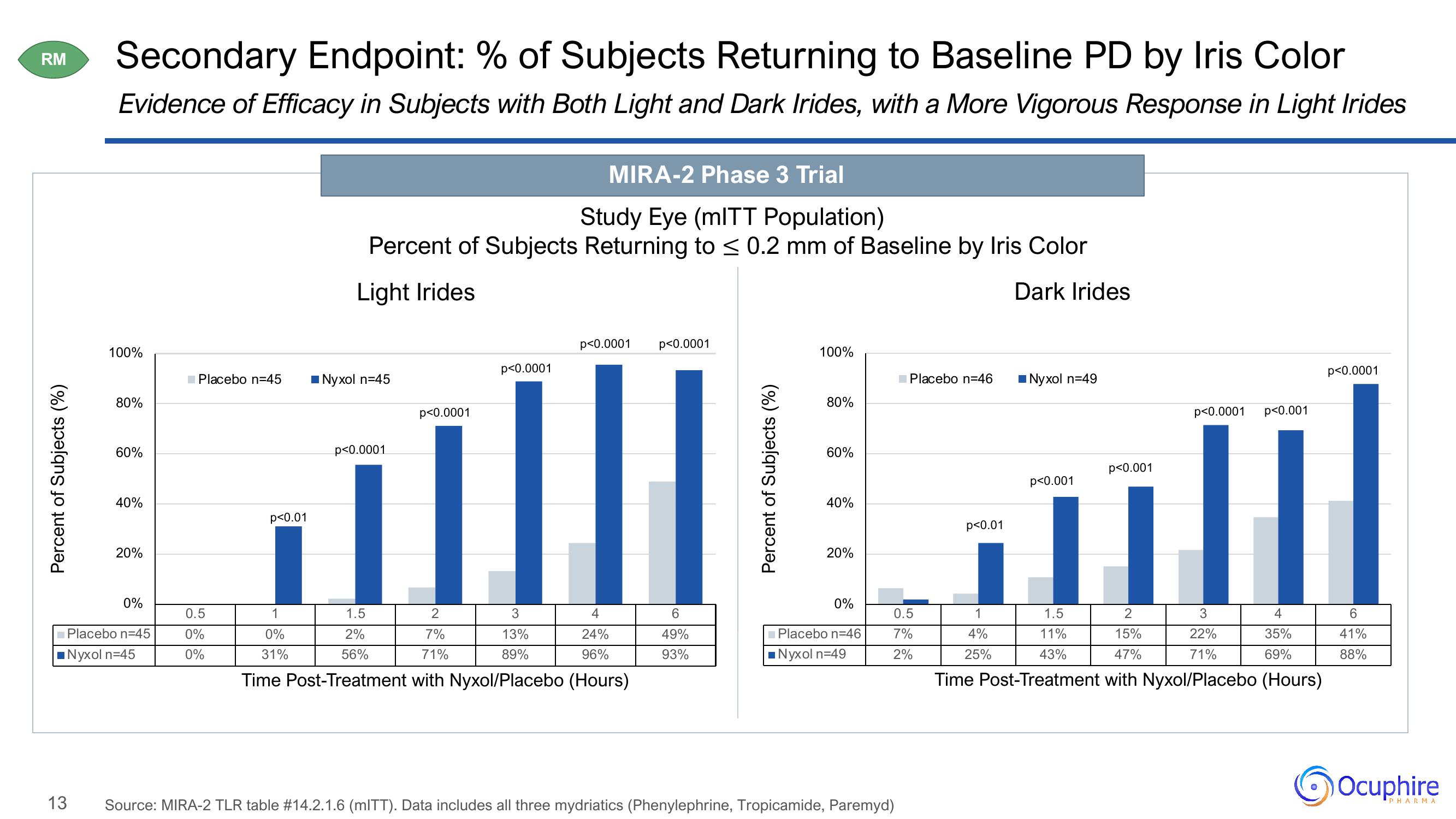
Secondary Endpoint: % of Subjects Returning to Baseline PD by Iris Color
Evidence of Efficacy in Subjects with Both Light and Dark Irides, with a More Vigorous Response in Light Irides
13
100%
80%
60%
40%
20%
0%
Placebo n=45
Nyxol n=45
Placebo n=45
0.5
0%
0%
p<0.01
MIRA-2 Phase 3 Trial
Study Eye (mITT Population)
Percent of Subjects Returning to ≤ 0.2 mm of Baseline by Iris Color
Light Irides
Dark Irides
1
0%
31%
Nyxol n=45
p<0.0001
p<0.0001
p<0.0001
p<0.0001 p<0.0001
H
1.5
2
4
2%
7%
3
13%
89%
24%
96%
56%
71%
Time Post-Treatment with Nyxol/Placebo (Hours)
6
49%
93%
100%
80%
60%
40%
20%
0%
Placebo n=46
■Nyxol n=49
Placebo n=46
0.5
7%
2%
Source: MIRA-2 TLR table #14.2.1.6 (mITT). Data includes all three mydriatics (Phenylephrine, Tropicamide, Paremyd)
p<0.01
Nyxol n=49
1
4%
25%
p<0.001
11
2
3
15%
22%
47%
71%
Time Post-Treatment with Nyxol/Placebo (Hours)
p<0.001
1.5
11%
43%
p<0.0001 p<0.001
4
35%
69%
p<0.0001
6
41%
88%
Ocuphire
PHARMAView entire presentation