AstraZeneca Results Presentation Deck
CEO Opening Remarks
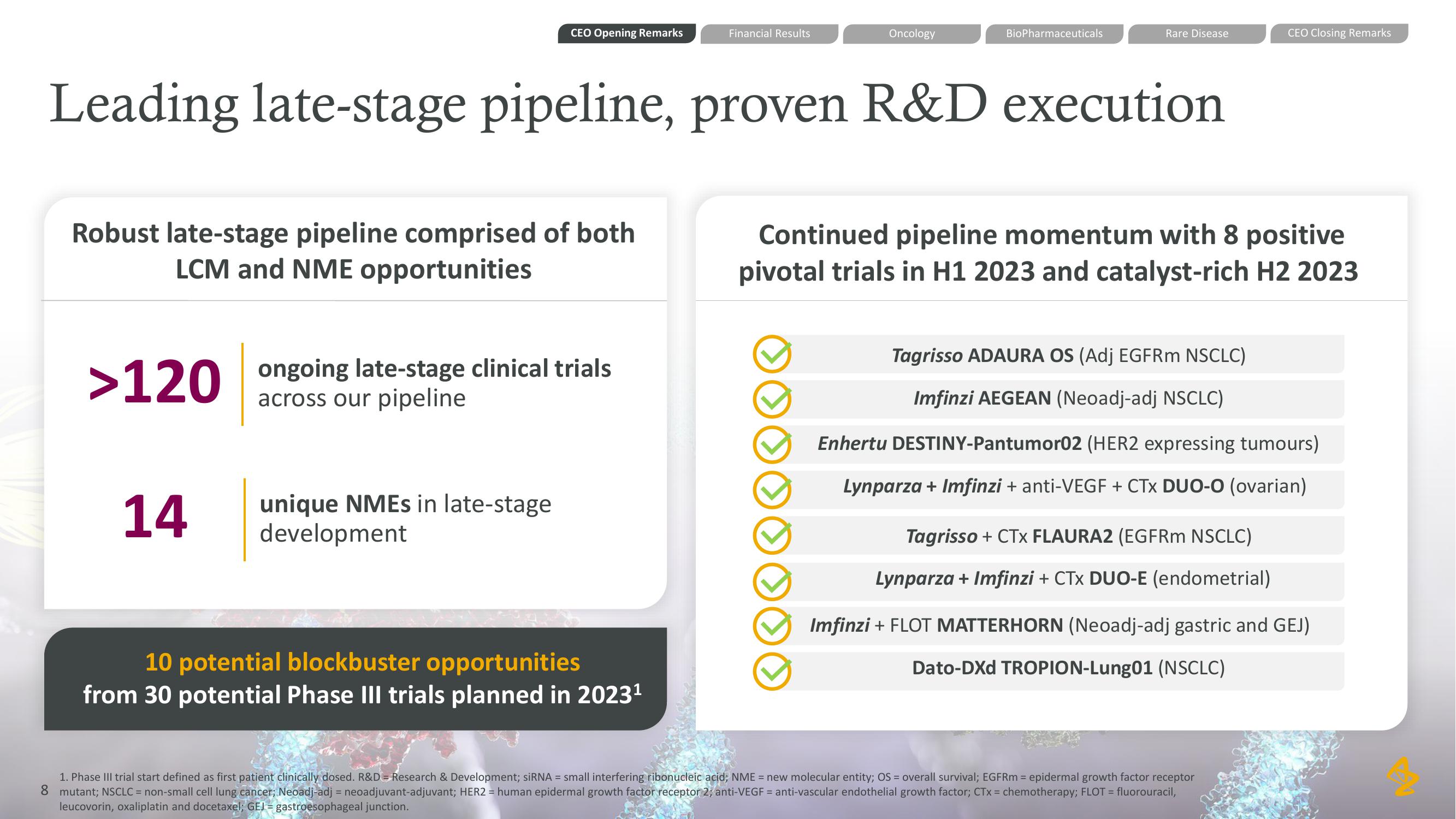
Robust late-stage pipeline comprised of both
LCM and NME opportunities
>120 ongoing late-stage clinical trials
across our pipeline
14
unique NMEs in late-stage
development
Leading late-stage pipeline, proven R&D execution
Financial Results
10 potential blockbuster opportunities
from 30 potential Phase III trials planned in 2023¹
Oncology
BioPharmaceuticals
Rare Disease
Continued pipeline momentum with 8 positive
pivotal trials in H1 2023 and catalyst-rich H2 2023
CEO Closing Remarks
Tagrisso ADAURA OS (Adj EGFRm NSCLC)
Imfinzi AEGEAN (Neoadj-adj NSCLC)
Enhertu DESTINY-Pantumor02 (HER2 expressing tumours)
Lynparza + Imfinzi + anti-VEGF + CTX DUO-O (ovarian)
Tagrisso + CTX FLAURA2 (EGFRm NSCLC)
Lynparza + Imfinzi + CTX DUO-E (endometrial)
Imfinzi + FLOT MATTERHORN (Neoadj-adj gastric and GEJ)
Dato-DXD TROPION-Lung01 (NSCLC)
1. Phase III trial start defined as first patient clinically dosed. R&D = Research & Development; siRNA = small interfering ribonucleic acid; NME = new molecular entity; OS = overall survival; EGFRm = epidermal growth factor receptor
8 mutant; NSCLC = non-small cell lung cancer; Neoadj-adj = neoadjuvant-adjuvant; HER2 = human epidermal growth factor receptor 2; anti-VEGF = anti-vascular endothelial growth factor; CTX = chemotherapy; FLOT = fluorouracil,
leucovorin, oxaliplatin and docetaxel; GEJ = gastroesophageal junction.View entire presentation