Genelux Investor Presentation Deck
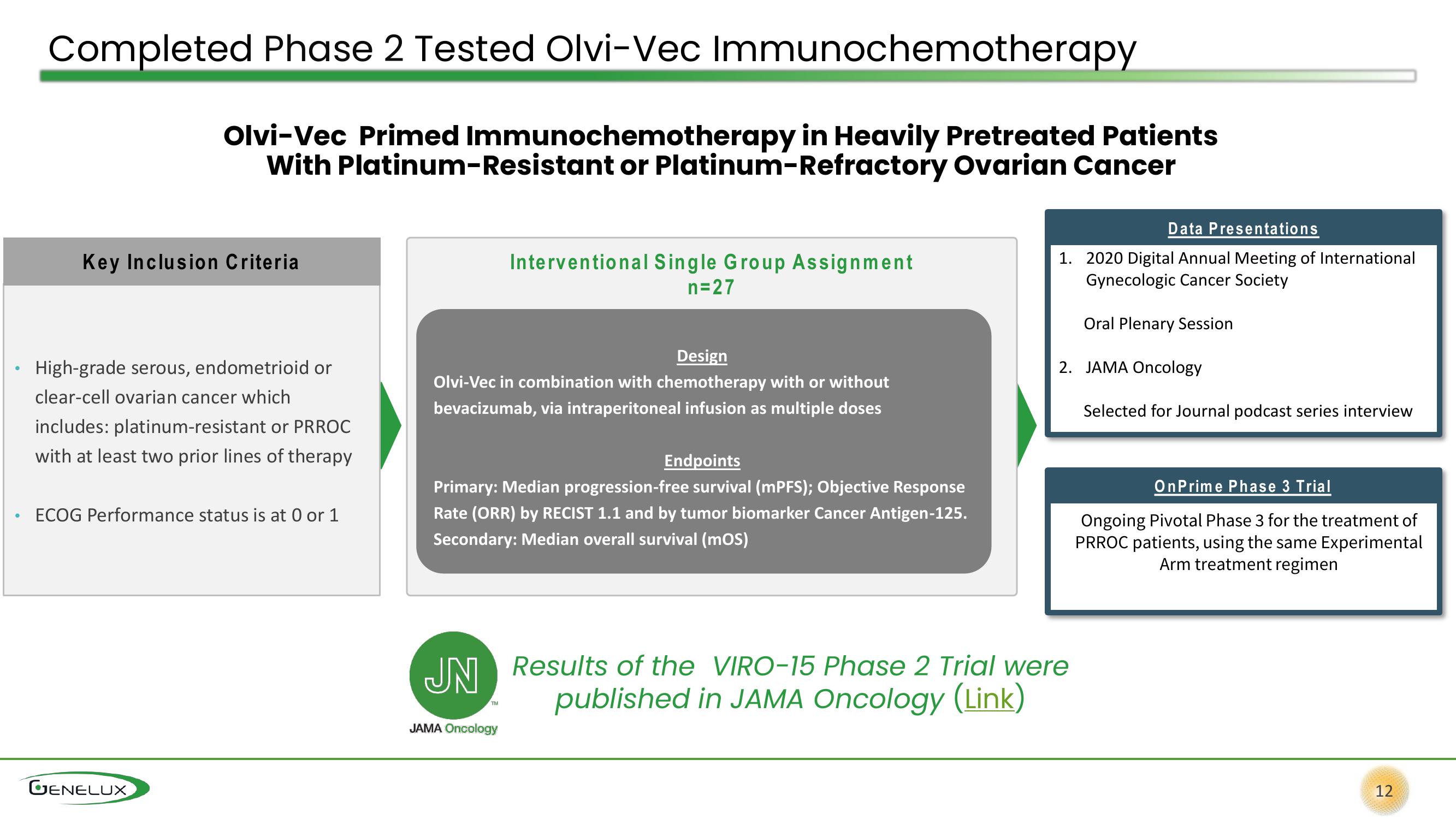
Completed Phase 2 Tested Olvi-Vec Immunochemotherapy
Olvi-Vec Primed Immunochemotherapy in Heavily Pretreated Patients
With Platinum-Resistant or Platinum-Refractory Ovarian Cancer
Key Inclusion Criteria
High-grade serous, endometrioid or
clear-cell ovarian cancer which
includes: platinum-resistant or PRROC
with at least two prior lines of therapy
ECOG Performance status is at 0 or 1
GENELUX
Design
Olvi-Vec in combination with chemotherapy with or without
bevacizumab, via intraperitoneal infusion as multiple doses
Interventional Single Group Assignment
n=27
Endpoints
Primary: Median progression-free survival (mPFS); Objective Response
Rate (ORR) by RECIST 1.1 and by tumor biomarker Cancer Antigen-125.
Secondary: Median overall survival (MOS)
TM
JAMA Oncology
Data Presentations
1. 2020 Digital Annual Meeting of International
Gynecologic Cancer Society
UN Results of the VIRO-15 Phase 2 Trial were
published in JAMA Oncology (Link)
Oral Plenary Session
2. JAMA Oncology
Selected for Journal podcast series interview
OnPrime Phase 3 Trial
Ongoing Pivotal Phase 3 for the treatment of
PRROC patients, using the same Experimental
Arm treatment regimen
12View entire presentation