Imara M&A
Concentration (ng/mL)
10000
1000
100-
10
0.1-
0
ELVN-002 Achieved a Wide Safety Margin in Preclinical Species
=
CHI
•
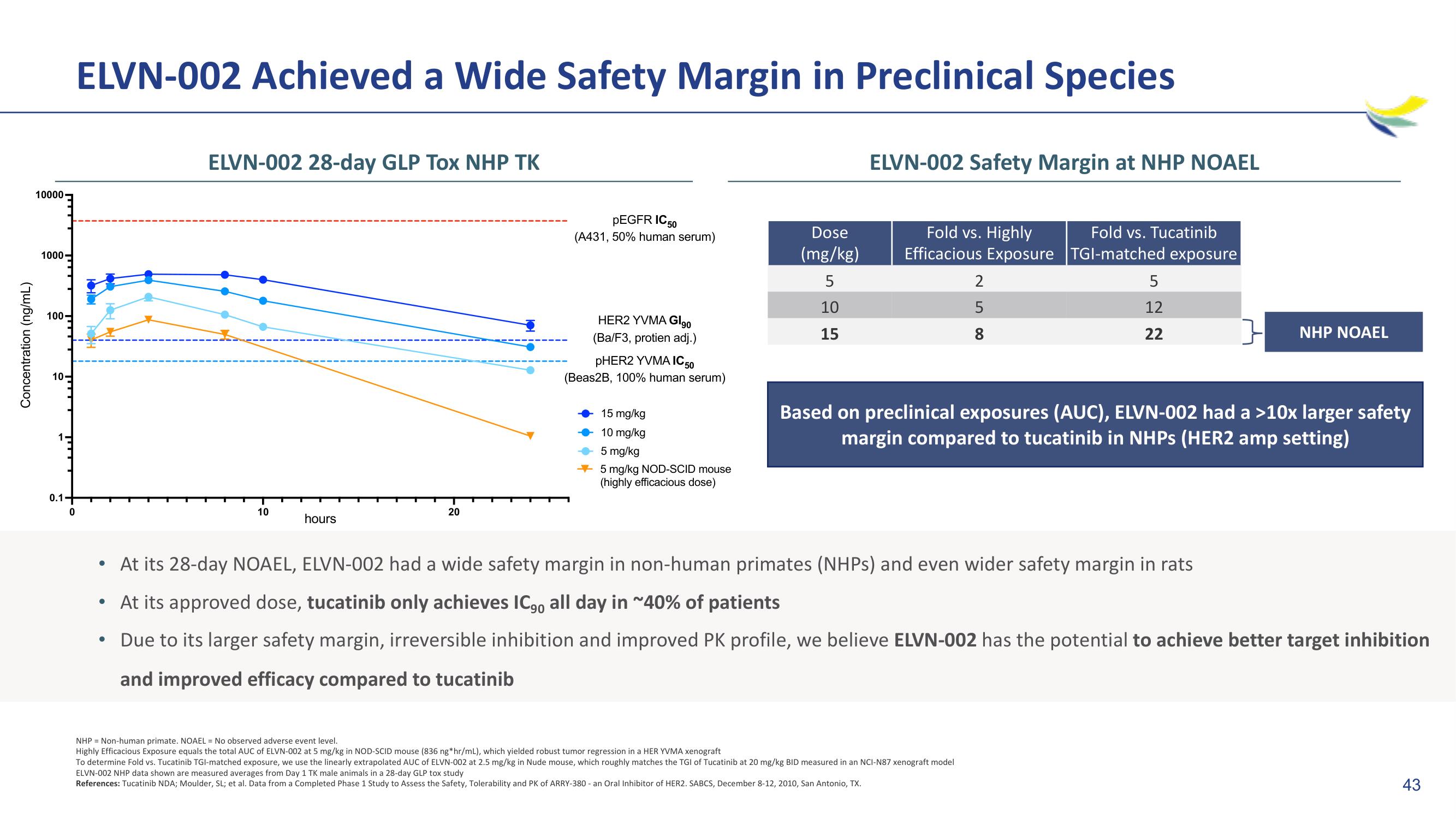
ELVN-002 28-day GLP Tox NHP TK
●
10
hours
20
pEGFR IC 50
(A431, 50% human serum)
HER2 YVMA GI90
(Ba/F3, protien adj.)
pHER2 YVMA IC 50
(Beas2B, 100% human serum)
15 mg/kg
10 mg/kg
5 mg/kg
5 mg/kg NOD-SCID mouse
(highly efficacious dose)
Dose
(mg/kg)
5
10
15
ELVN-002 Safety Margin at NHP NOAEL
Fold vs. Highly
Fold vs. Tucatinib
Efficacious Exposure TGI-matched exposure
2
5
8
5
12
22
Based on preclinical exposures (AUC), ELVN-002 had a >10x larger safety
margin compared to tucatinib in NHPs (HER2 amp setting)
At its 28-day NOAEL, ELVN-002 had a wide safety margin in non-human primates (NHPs) and even wider safety margin in rats
NHP = Non-human primate. NOAEL = No observed adverse event level.
Highly Efficacious Exposure equals the total AUC of ELVN-002 at 5 mg/kg in NOD-SCID mouse (836 ng*hr/mL), which yielded robust tumor regression in a HER YVMA xenograft
To determine Fold vs. Tucatinib TGI-matched exposure, we use the linearly extrapolated AUC of ELVN-002 at 2.5 mg/kg in Nude mouse, which roughly matches the TGI of Tucatinib at 20 mg/kg BID measured in an NCI-N87 xenograft model
ELVN-002 NHP data shown are measured averages from Day 1 TK male animals in a 28-day GLP tox study
References: Tucatinib NDA; Moulder, SL; et al. Data from a Completed Phase 1 Study to Assess the Safety, Tolerability and PK of ARRY-380-an Oral Inhibitor of HER2. SABCS, December 8-12, 2010, San Antonio, TX.
NHP NOAEL
At its approved dose, tucatinib only achieves IC., all day in ~40% of patients
• Due to its larger safety margin, irreversible inhibition and improved PK profile, we believe ELVN-002 has the potential to achieve better target inhibition
and improved efficacy compared to tucatinib
43View entire presentation