Ocuphire Pharma Results Presentation Deck
8
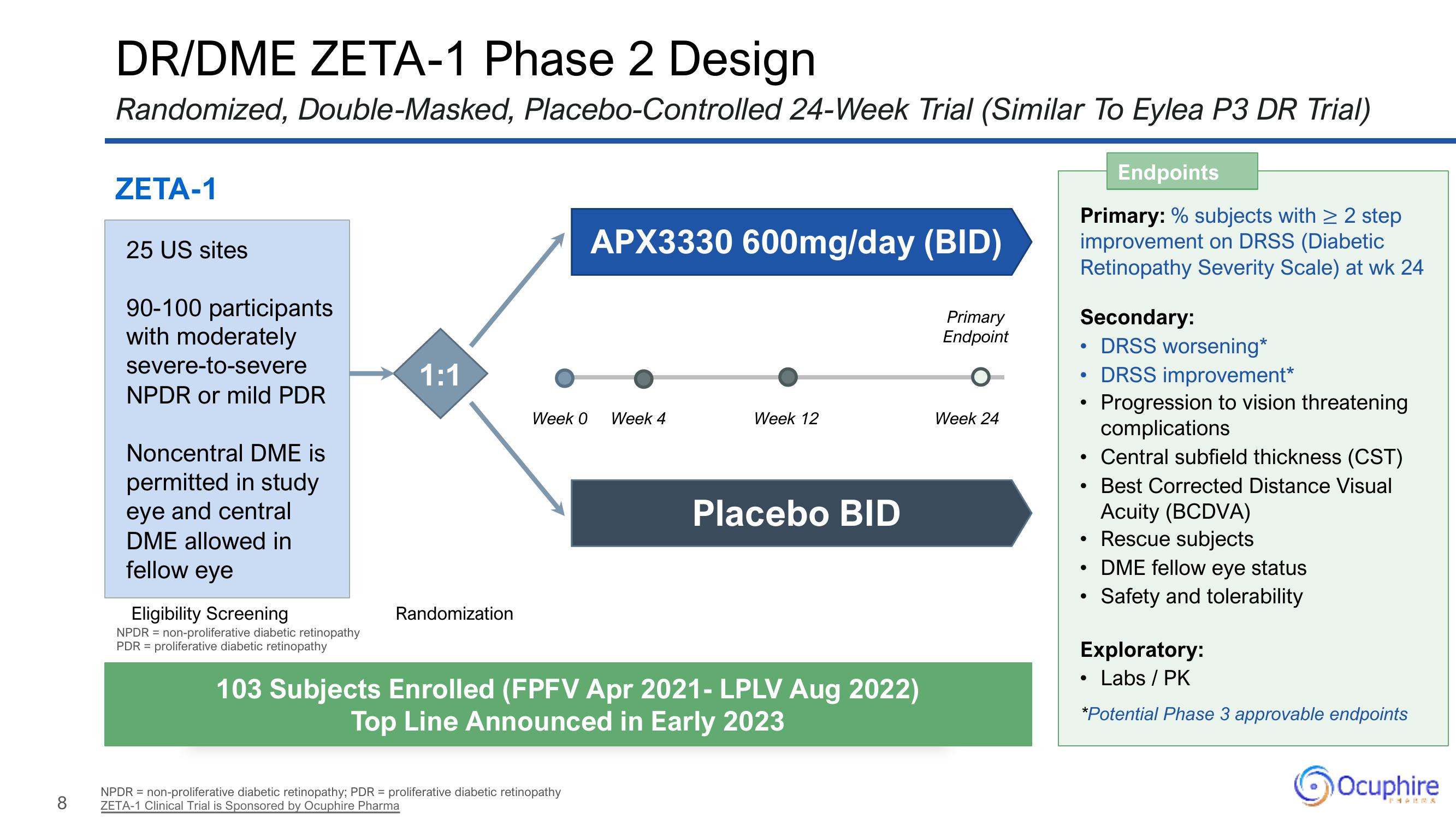
DR/DME ZETA-1 Phase 2 Design
Randomized, Double-Masked, Placebo-Controlled 24-Week Trial (Similar To Eylea P3 DR Trial)
ZETA-1
25 US sites
90-100 participants
with moderately
severe-to-severe
NPDR or mild PDR
Noncentral DME is
permitted in study
eye and central
DME allowed in
fellow eye
Eligibility Screening
NPDR = non-proliferative diabetic retinopathy
PDR = proliferative diabetic retinopathy
1:1
Randomization
Week 0
APX3330 600mg/day (BID)
Primary
Endpoint
NPDR = non-proliferative diabetic retinopathy; PDR = proliferative diabetic retinopathy
ZETA-1 Clinical Trial is Sponsored by Ocuphire Pharma
Week 4
Week 12
Placebo BID
103 Subjects Enrolled (FPFV Apr 2021- LPLV Aug 2022)
Top Line Announced in Early 2023
Week 24
Endpoints
Primary: % subjects with ≥ 2 step
improvement on DRSS (Diabetic
Retinopathy Severity Scale) at wk 24
Secondary:
●
DRSS worsening*
DRSS improvement*
Progression to vision threatening
complications
Central subfield thickness (CST)
Best Corrected Distance Visual
Acuity (BCDVA)
Rescue subjects
• DME fellow eye status
Safety and tolerability
Exploratory:
Labs / PK
*Potential Phase 3 approvable endpoints
OcuphireView entire presentation