Bionomics Investor Presentation Deck
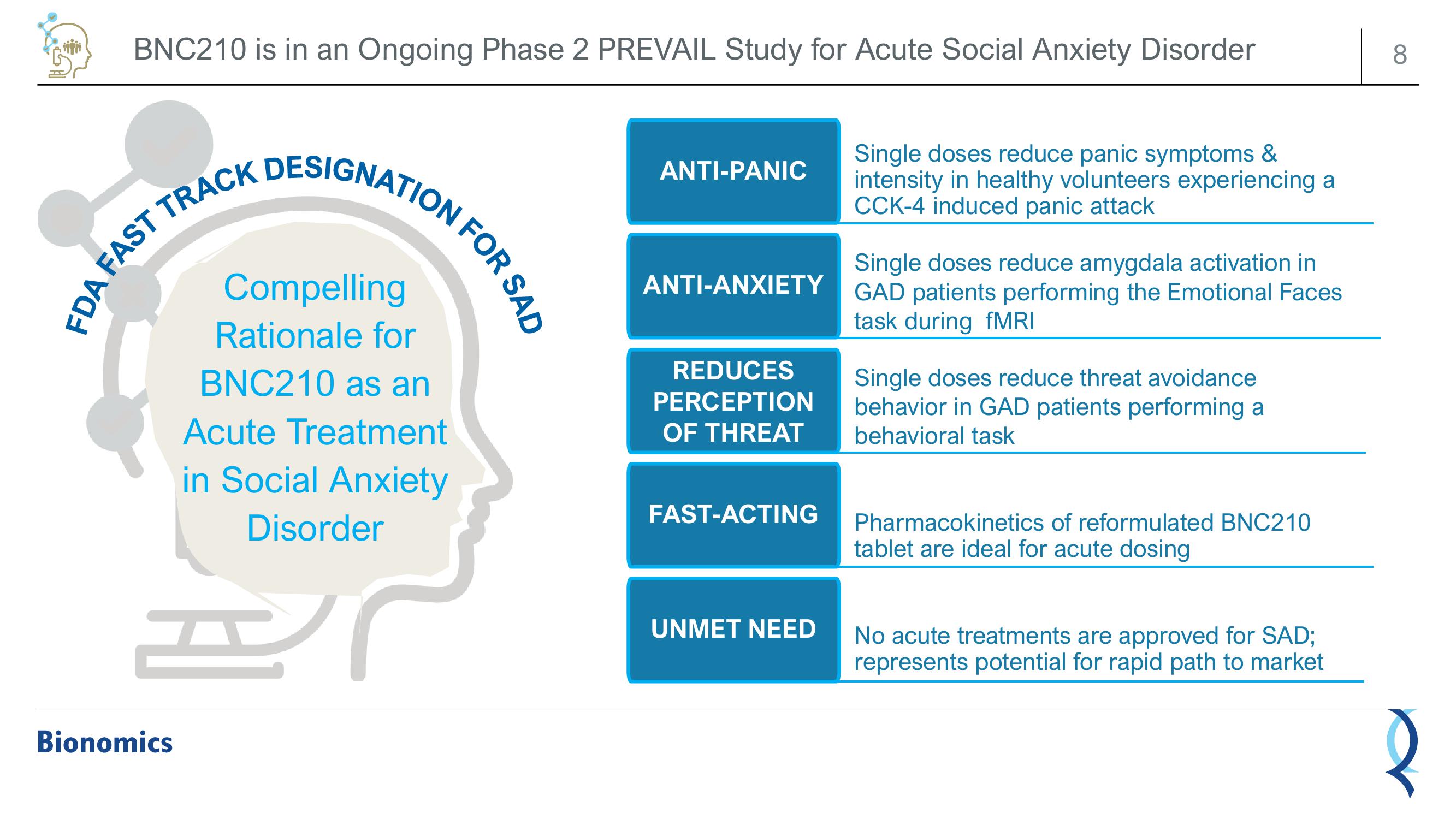
BNC210 is in an Ongoing Phase 2 PREVAIL Study for Acute Social Anxiety Disorder
FDA FAST TRACK DESIGNATION F
Compelling
Rationale for
BNC210 as an
Acute Treatment
in Social Anxiety
Disorder
A
Bionomics
FOR SAD
ANTI-PANIC
ANTI-ANXIETY
REDUCES
PERCEPTION
OF THREAT
FAST-ACTING
UNMET NEED
Single doses reduce panic symptoms &
intensity in healthy volunteers experiencing a
CCK-4 induced panic attack
Single doses reduce amygdala activation in
GAD patients performing the Emotional Faces
task during fMRI
Single doses reduce threat avoidance
behavior in GAD patients performing a
behavioral task
Pharmacokinetics of reformulated BNC210
tablet are ideal for acute dosing
No acute treatments are approved for SAD;
represents potential for rapid path to market
8View entire presentation