AstraZeneca Investor Day Presentation Deck
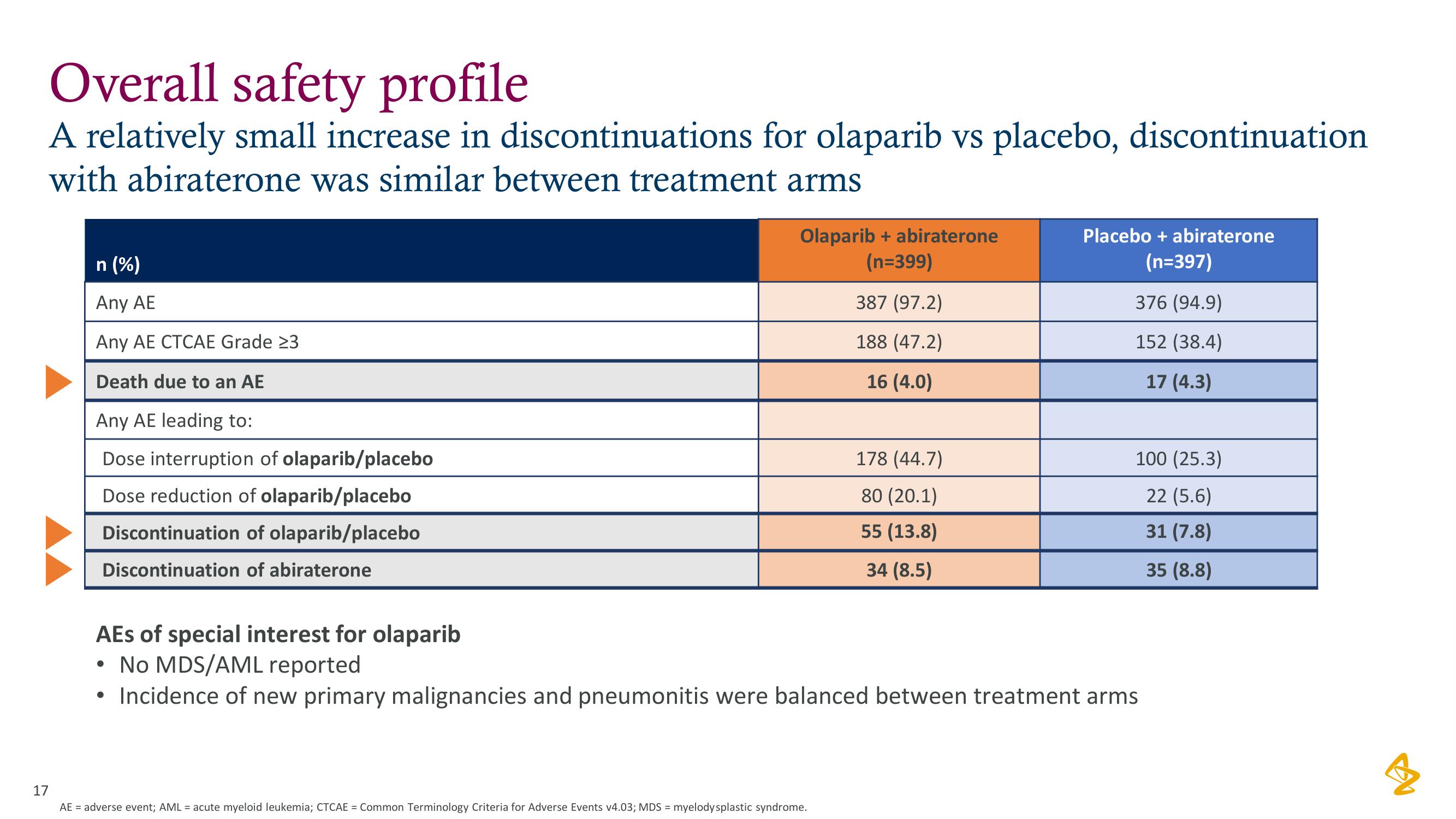
Overall safety profile
A relatively small increase in discontinuations for olaparib vs placebo, discontinuation
with abiraterone was similar between treatment arms
n (%)
Any AE
Any AE CTCAE Grade 23
Death due to an AE
Any AE leading to:
Dose interruption of olaparib/placebo
Dose reduction of olaparib/placebo
Discontinuation of olaparib/placebo
Discontinuation of abiraterone
Olaparib + abiraterone
(n=399)
387 (97.2)
188 (47.2)
16 (4.0)
17
AE = adverse event; AML = acute myeloid leukemia; CTCAE = Common Terminology Criteria for Adverse Events v4.03; MDS = myelodysplastic syndrome.
178 (44.7)
80 (20.1)
55 (13.8)
34 (8.5)
Placebo + abiraterone
(n=397)
376 (94.9)
152 (38.4)
17 (4.3)
100 (25.3)
22 (5.6)
31 (7.8)
35 (8.8)
AEs of special interest for olaparib
• No MDS/AML reported
Incidence of new primary malignancies and pneumonitis were balanced between treatment arms
BView entire presentation