Comera Investor Presentation Deck
LIFE SCIENCES
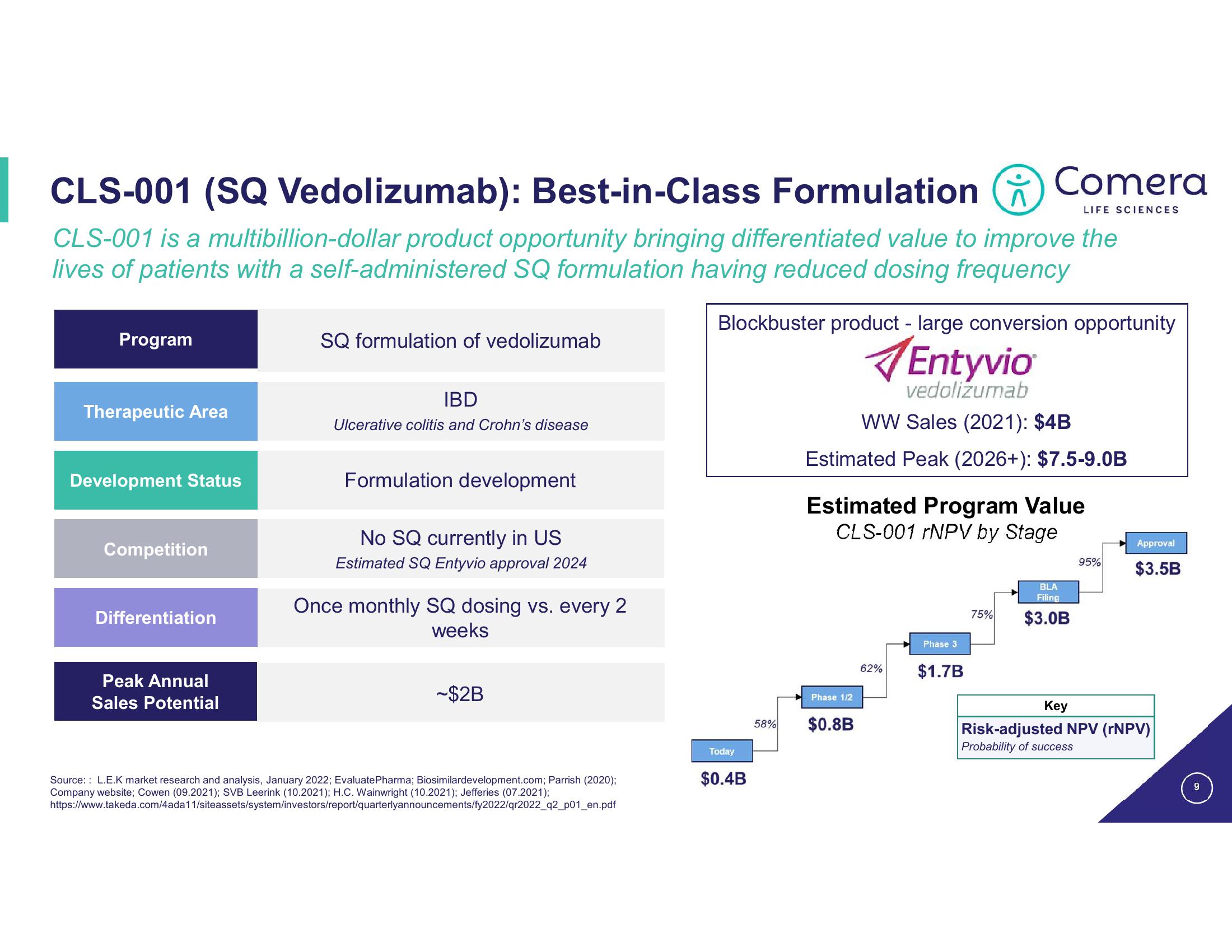
CLS-001 (SQ Vedolizumab): Best-in-Class Formulation
CLS-001 is a multibillion-dollar product opportunity bringing differentiated value to improve the
lives of patients with a self-administered SQ formulation having reduced dosing frequency
Program
Therapeutic Area
Development Status
Competition
Differentiation
Peak Annual
Sales Potential
SQ formulation of vedolizumab
IBD
Ulcerative colitis and Crohn's disease
Formulation development
No SQ currently in US
Estimated SQ Entyvio approval 2024
Once monthly SQ dosing vs. every 2
weeks
-$2B
Source: L.E.K market research and analysis, January 2022; Evaluate Pharma; Biosimilardevelopment.com; Parrish (2020);
Company website; Cowen (09.2021); SVB Leerink (10.2021); H.C. Wainwright (10.2021); Jefferies (07.2021);
https://www.takeda.com/4ada11/siteassets/system/investors/report/quarterlyannouncements/fy2022/qr2022_q2_p01_en.pdf
Blockbuster product - large conversion opportunity
Entyvio®
vedolizumab
Today
$0.4B
58%
WW Sales (2021): $4B
Estimated Peak (2026+): $7.5-9.0B
Estimated Program Value
CLS-001 rNPV by Stage
Phase 1/2
$0.8B
Comera
62%
Phase 3
$1.7B
75%
BLA
Filing
$3.0B
Approval
95% $3.5B
Key
Risk-adjusted NPV (NPV)
Probability of success
9View entire presentation