ATAI Investor Presentation Deck
SUMMARY
OWNERSHIP 100%¹
PRODUCT
PHARMA-
COLOGY
PRODUCT
FEATURES
INDICATIONS
CURRENT
STATUS
INTELLECTUAL
PROPERTY
HIGHLIGHT
Dimethyltryptamine (DMT) in a buccal
transmucosal film (VLS-01), DMT is the active
psychedelic moiety in Ayahuasca
5-HT2A-R agonist
Rapid onset, sustained efficacy after single
dose, short duration of psychedelic effect
(~30 to 45 minutes)
Primary: Treatment Resistant Depression
Potential: Eating Disorders, Substance Use
Disorders
Phase 1 clinical trial initiated in mid-'22
Filed provisional on formulations of DMT
VLS-01 is designed to have an improved
duration of psychedelic effect while improving
tolerability
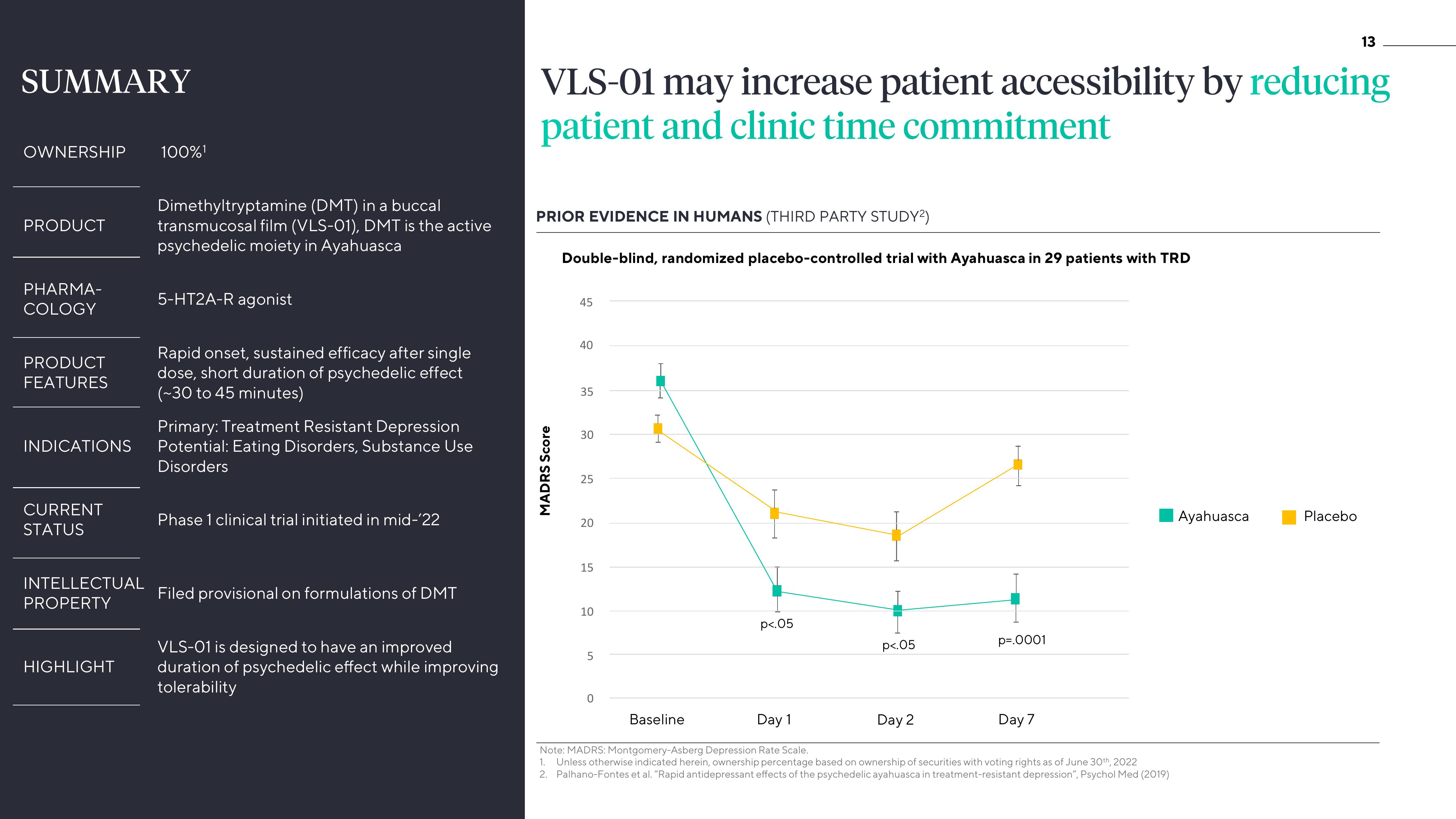
VLS-01 may increase patient accessibility by reducing
patient and clinic time commitment
PRIOR EVIDENCE IN HUMANS (THIRD PARTY STUDY²)
MADRS Score
Double-blind, randomized placebo-controlled trial with Ayahuasca in 29 patients with TRD
45
40
35
30
25
20
15
10
5
0
HA
Baseline
p<.05
Day 1
p<.05
Day 2
HH
p=.0001
Day 7
Note: MADRS: Montgomery-Asberg Depression Rate Scale.
1. Unless otherwise indicated herein, ownership percentage based on ownership of securities with voting rights as of June 30th, 2022
2. Palhano-Fontes et al. "Rapid antidepressant effects of the psychedelic ayahuasca in treatment-resistant depression", Psychol Med (2019)
Ayahuasca
13
PlaceboView entire presentation