Centessa IPO Presentation Deck
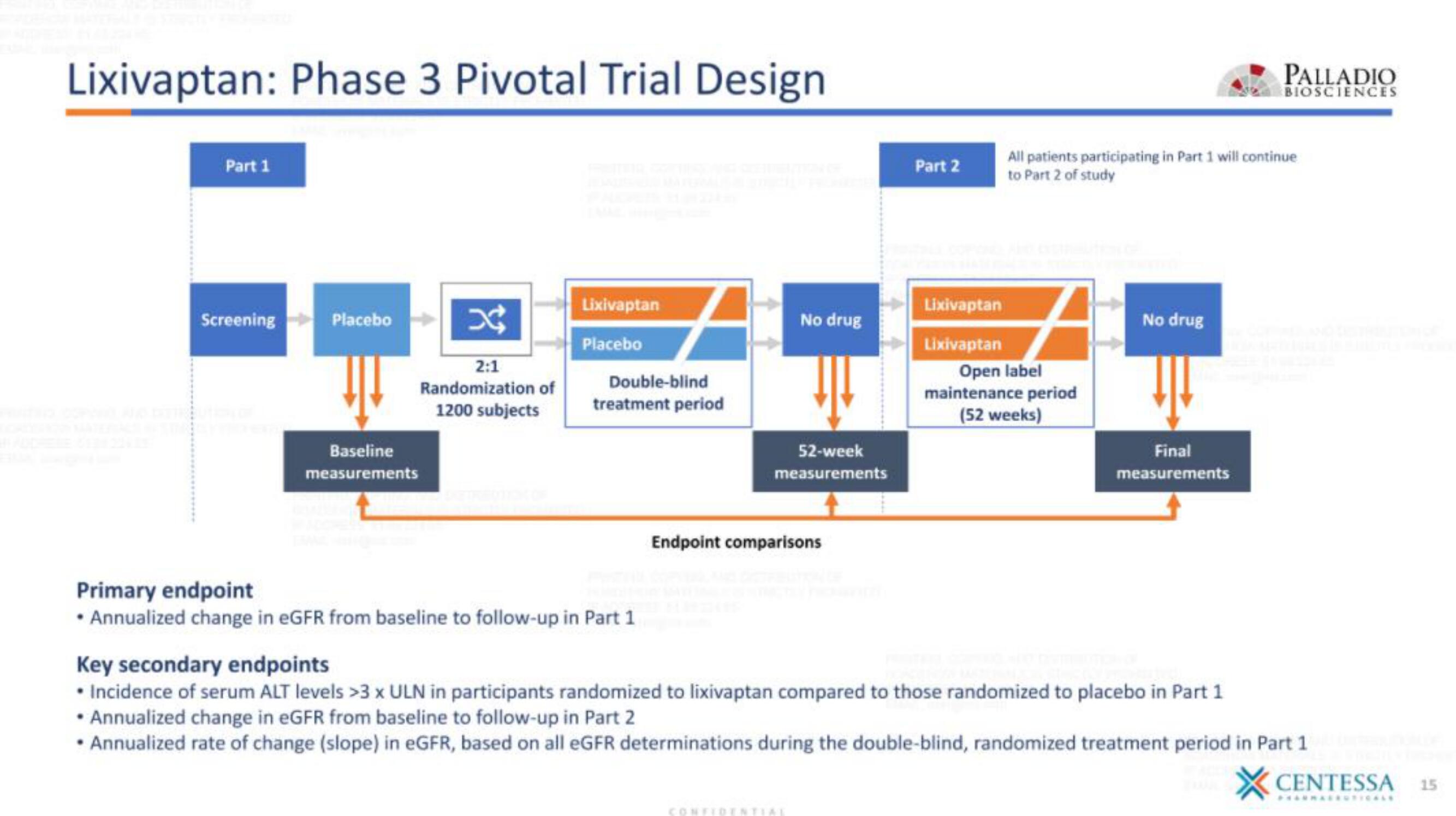
Lixivaptan: Phase 3 Pivotal Trial Design
Part 1
Screening Placebo
Baseline
measurements
x
2:1
Randomization of
1200 subjects
Lixivaptan
Placebo
H
Double-blind
treatment period
Primary endpoint
• Annualized change in eGFR from baseline to follow-up in Part 1
No drug
52-week
measurements
Endpoint comparisons
Part 2
Lixivaptan
Lixivaptan
All patients participating in Part 1 will continue
to Part 2 of study
Open label
maintenance period
(52 weeks)
No drug
PALLADIO
BIOSCIENCES
Final
measurements
Key secondary endpoints
• Incidence of serum ALT levels 3 x ULN in participants randomized to lixivaptan compared to those randomized to placebo in Part 1
Annualized change in eGFR from baseline to follow-up in Part 2
• Annualized rate of change (slope) in eGFR, based on all eGFR determinations during the double-blind, randomized treatment period in Part 1
X CENTESSA 15View entire presentation