Ocuphire Pharma Investor Updates
P
21
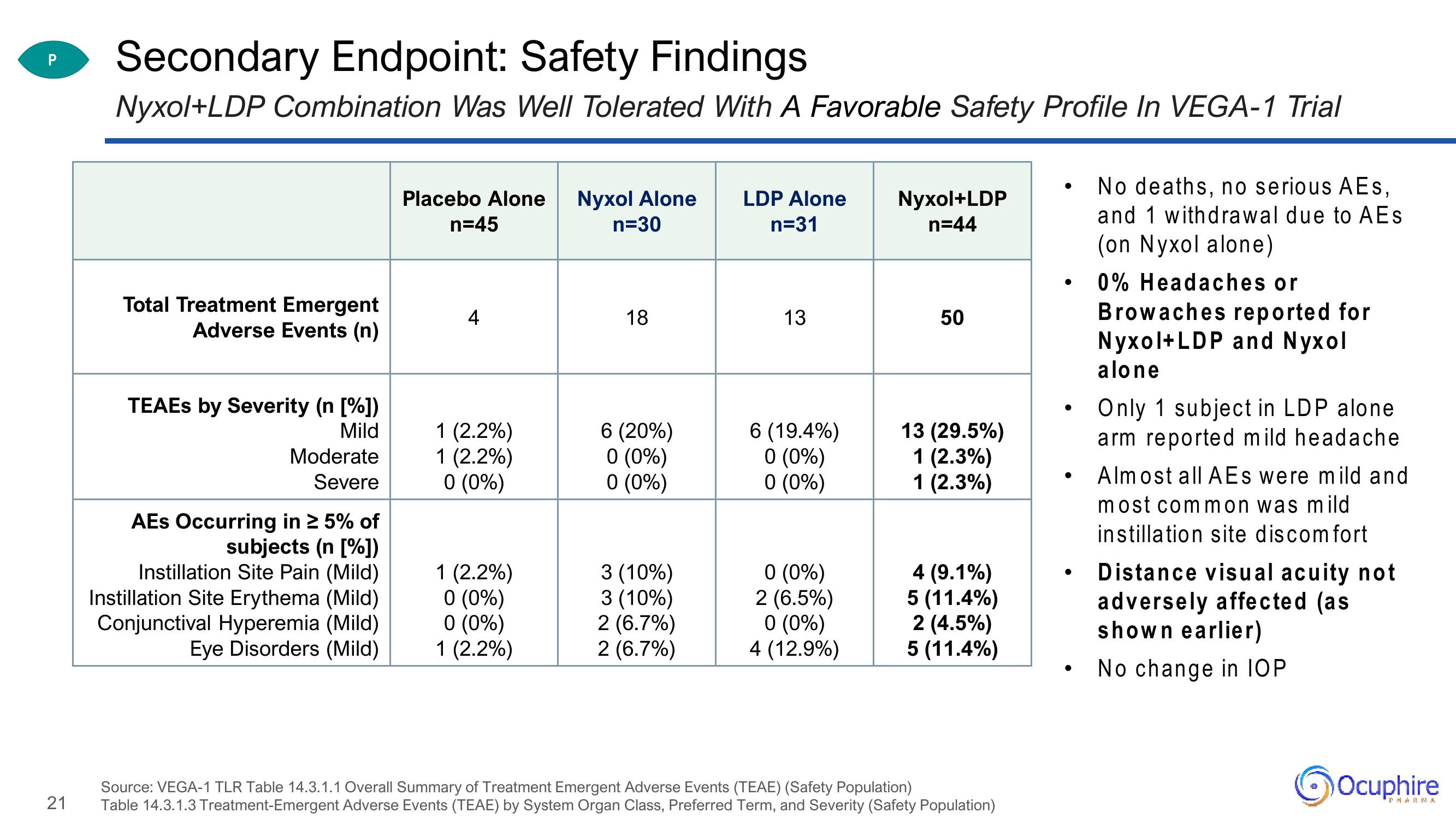
Secondary Endpoint: Safety Findings
Nyxol+LDP Combination Was Well Tolerated With A Favorable Safety Profile In VEGA-1 Trial
Total Treatment Emergent
Adverse Events (n)
TEAES by Severity (n [%])
Mild
Moderate
Severe
AES Occurring in ≥ 5% of
subjects (n [%])
Instillation Site Pain (Mild)
Instillation Site Erythema (Mild)
Conjunctival Hyperemia (Mild)
Eye Disorders (Mild)
Placebo Alone Nyxol Alone
n=45
n=30
4
1 (2.2%)
1 (2.2%)
0 (0%)
1 (2.2%)
0 (0%)
0 (0%)
1 (2.2%)
18
6 (20%)
0 (0%)
0 (0%)
3 (10%)
3 (10%)
2 (6.7%)
2 (6.7%)
LDP Alone
n=31
13
6 (19.4%)
0 (0%)
0 (0%)
0 (0%)
2 (6.5%)
0 (0%)
4 (12.9%)
Nyxol+LDP
n=44
50
13 (29.5%)
1 (2.3%)
1 (2.3%)
4 (9.1%)
5 (11.4%)
2 (4.5%)
5 (11.4%)
Source: VEGA-1 TLR Table 14.3.1.1 Overall Summary of Treatment Emergent Adverse Events (TEAE) (Safety Population)
Table 14.3.1.3 Treatment-Emergent Adverse Events (TEAE) by System Organ Class, Preferred Term, and Severity (Safety Population)
●
●
●
No deaths, no serious AEs,
and 1 withdrawal due to AEs
(on Nyxol alone)
0% Headaches or
Browaches reported for
Nyxol+LDP and Nyxol
alone
Only 1 subject in LDP alone
arm reported mild headache
Almost all AEs were mild and
most common was mild
instillation site discomfort
Distance visual acuity not
adversely affected (as
shown earlier)
No change in IOP
Ocuphire
PHARMAView entire presentation