Imara M&A
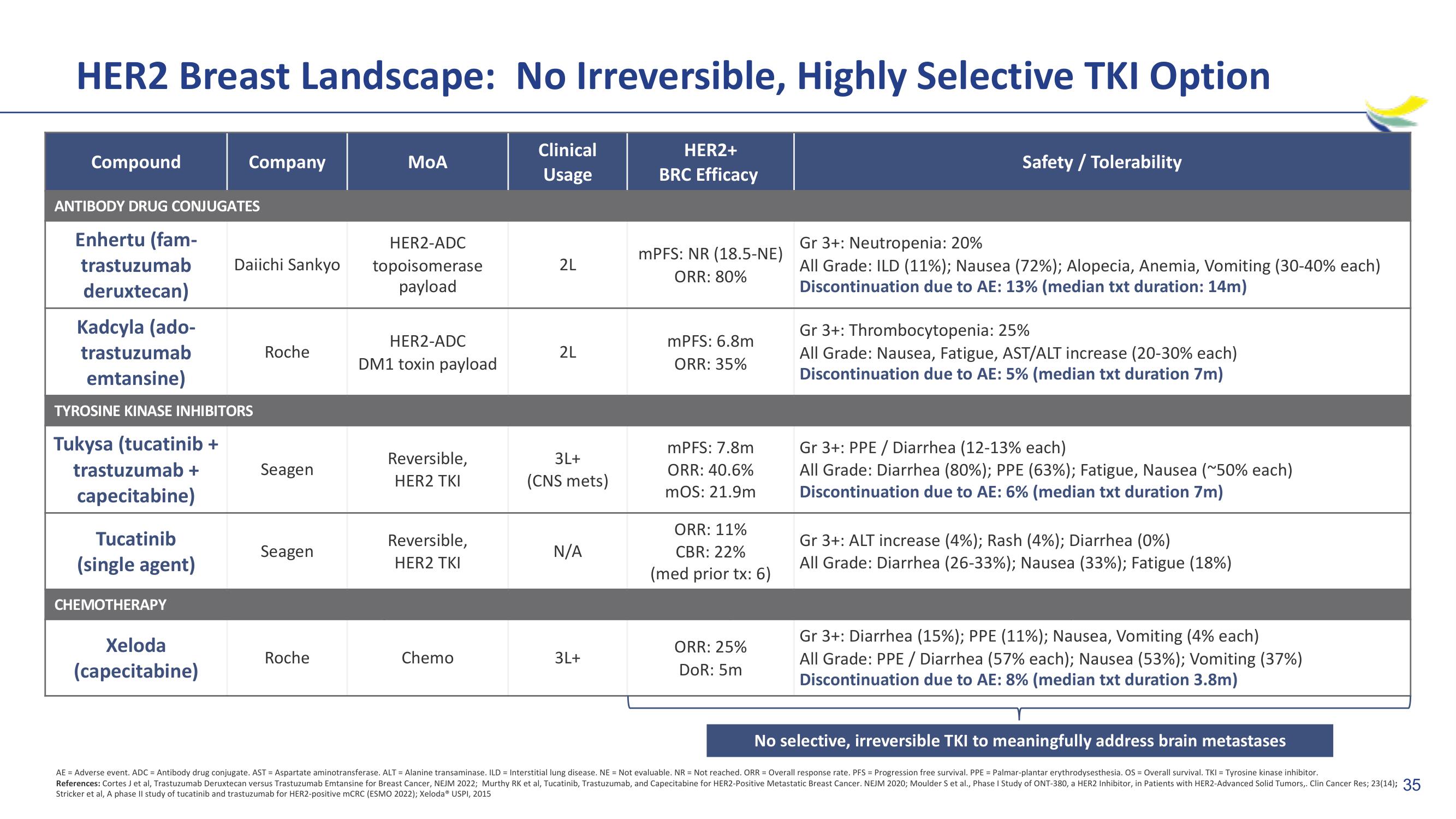
HER2 Breast Landscape: No Irreversible, Highly Selective TKI Option
Clinical
Usage
HER2+
BRC Efficacy
Compound
ANTIBODY DRUG CONJUGATES
Enhertu (fam-
trastuzumab
deruxtecan)
Kadcyla (ado-
trastuzumab
emtansine)
trastuzumab +
capecitabine)
Tucatinib
(single agent)
Company
TYROSINE KINASE INHIBITORS
Tukysa (tucatinib +
CHEMOTHERAPY
Xeloda
(capecitabine)
Daiichi Sankyo
Roche
Seagen
Seagen
Roche
MoA
HER2-ADC
topoisomerase
payload
HER2-ADC
DM1 toxin payload
Reversible,
HER2 TKI
Reversible,
HER2 TKI
Chemo
2L
2L
3L+
(CNS mets)
N/A
3L+
mPFS: NR (18.5-NE)
ORR: 80%
mPFS: 6.8m
ORR: 35%
mPFS: 7.8m
ORR: 40.6%
mOS: 21.9m
ORR: 11%
CBR: 22%
(med prior tx: 6)
ORR: 25%
DoR: 5m
Safety / Tolerability
Gr 3+: Neutropenia: 20%
All Grade: ILD (11%); Nausea (72%); Alopecia, Anemia, Vomiting (30-40% each)
Discontinuation due to AE: 13% (median txt duration: 14m)
Gr 3+: Thrombocytopenia: 25%
All Grade: Nausea, Fatigue, AST/ALT increase (20-30% each)
Discontinuation due to AE: 5% (median txt duration 7m)
Gr 3+: PPE / Diarrhea (12-13% each)
All Grade: Diarrhea (80%) ; PPE (63%) ; Fatigue, Nausea (~50% each)
Discontinuation due to AE: 6% (median txt duration 7m)
Gr 3+: ALT increase (4%) ; Rash (4%) ; Diarrhea (0%)
All Grade: Diarrhea (26-33%); Nausea (33%); Fatigue (18%)
Gr 3+: Diarrhea (15%); PPE (11%); Nausea, Vomiting (4% each)
All Grade: PPE / Diarrhea (57% each); Nausea (53%); Vomiting (37%)
Discontinuation due to AE: 8% (median txt duration 3.8m)
No selective, irreversible TKI to meaningfully address brain metastases
AE = Adverse event. ADC = Antibody drug conjugate. AST= Aspartate aminotransferase. ALT= Alanine transaminase. ILD = Interstitial lung disease. NE = Not evaluable. NR = Not reached. ORR = Overall response rate. PFS = Progression free survival. PPE = Palmar-plantar erythrodysesthesia. OS = Overall survival. TKI = Tyrosine kinase inhibitor.
References: Cortes J et al, Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer, NEJM 2022; Murthy RK et al, Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer. NEJM 2020; Moulder S et al., Phase I Study of ONT-380, a HER2 Inhibitor, in Patients with HER2-Advanced Solid Tumors,. Clin Cancer Res; 23(14); 35
Stricker et al, A phase II study of tucatinib and trastuzumab for HER2-positive mCRC (ESMO 2022); Xeloda USPI, 2015View entire presentation