MaxCyte IPO Presentation Deck
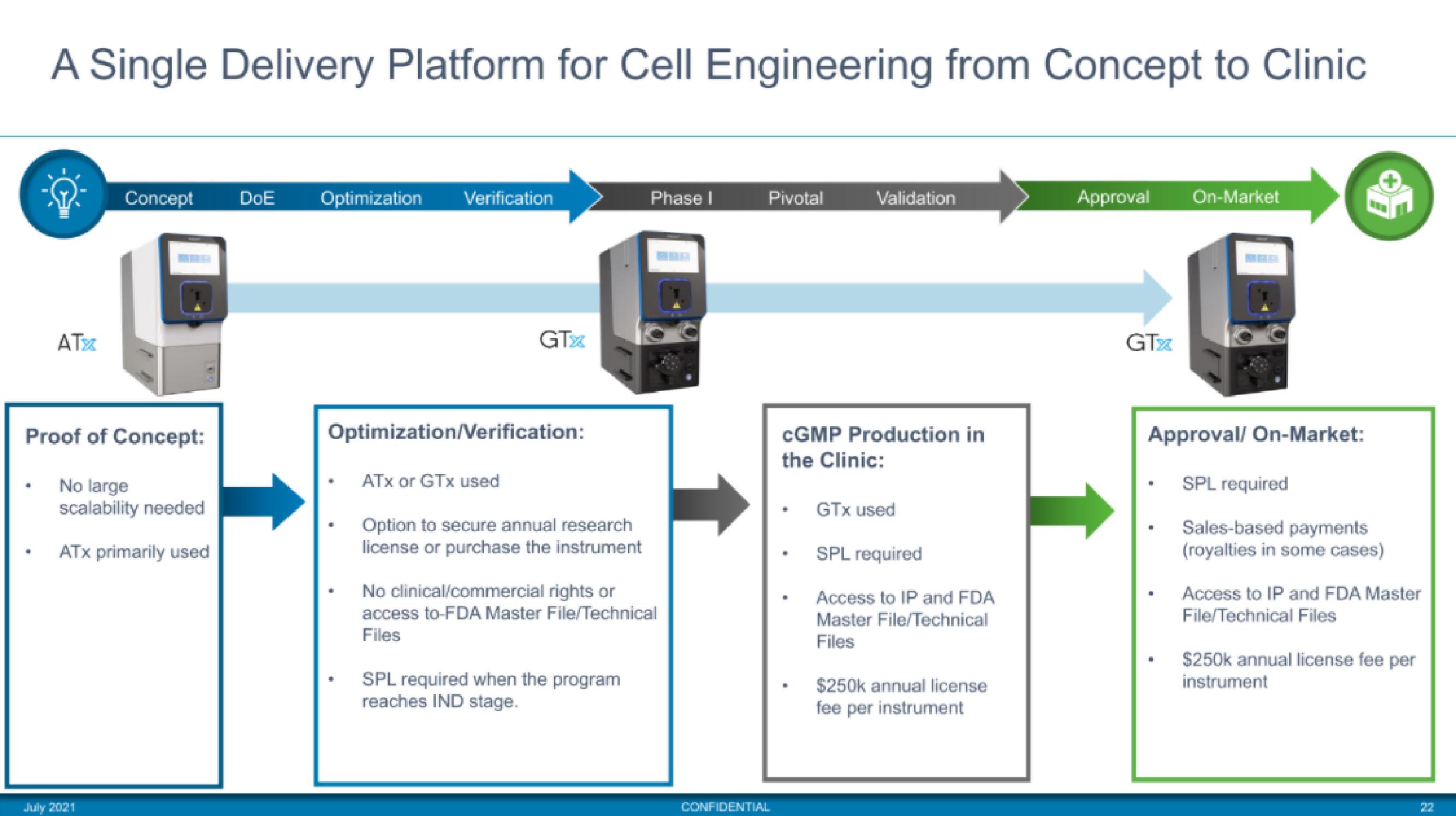
A Single Delivery Platform for Cell Engineering from Concept to Clinic
ATX
Concept DOE Optimization Verification
Proof of Concept:
No large
scalability needed
ATX primarily used
July 2021
GTX
Optimization/Verification:
ATX or GTX used
Option to secure annual research
license or purchase the instrument
Phase I
No clinical/commercial rights or
access to-FDA Master File/Technical
Files
SPL required when the program
reaches IND stage.
Pivotal
CONFIDENTIAL
Validation
CGMP Production in
the Clinic:
GTX used
SPL required
Access to IP and FDA
Master File/Technical
Files
$250k annual license
fee per instrument
Approval On-Market
GTX
Approval/ On-Market:
SPL required
Sales-based payments
(royalties in some cases)
Access to IP and FDA Master
File/Technical Files
$250k annual license fee per
instrumentView entire presentation