BioAtla IPO Presentation Deck
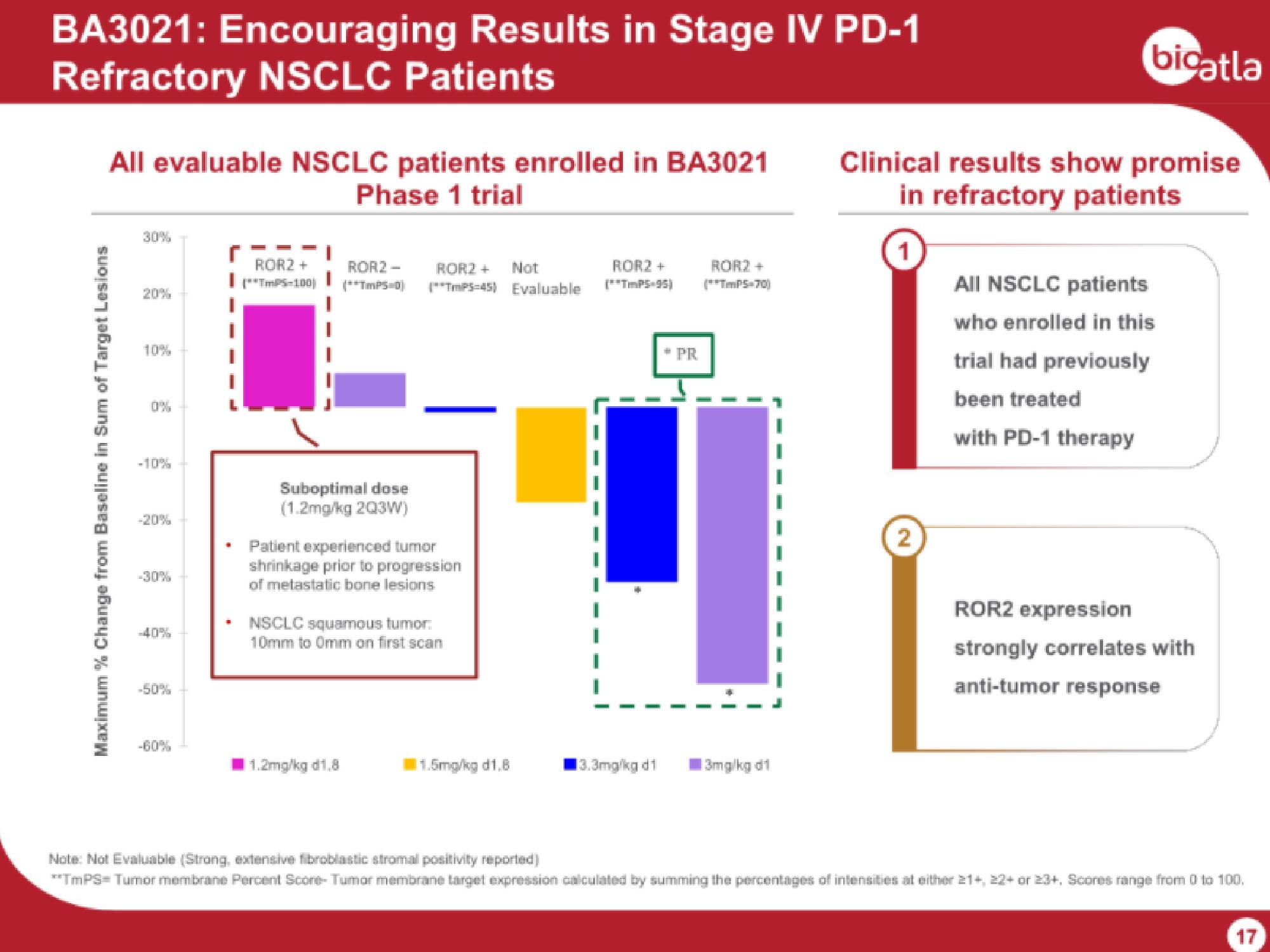
BA3021: Encouraging Results in Stage IV PD-1
Refractory NSCLC Patients
All evaluable NSCLC patients enrolled in BA3021
Phase 1 trial
Maximum % Change from Baseline in Sum of Target Lesions
30%
20%
10%
-10%
-20%
-30%
-40%
-50%
-60%
■
ROR2 +
**TmPS-100
ROR2-
(**TmPS D
Suboptimal dose
(1.2mg/kg 2Q3W)
ROR2 + Not
(**TmPS-45) Evaluable
Patient experienced tumor
shrinkage prior to progression
of metastatic bone lesions
1.2mg/kg d1,8
NSCLC squamous tumor:
10mm to 0mm on first scan
11.5mg/kg d1,8
ROR2 +
13.3mg/kg di
* PR
ROR2 +
(**TmPS-70)
3mg/kg di
bicatla
Clinical results show promise
in refractory patients
2
All NSCLC patients
who enrolled in this
trial had previously
been treated
with PD-1 therapy
ROR2 expression
strongly correlates with
anti-tumor response
Note: Not Evaluable (Strong, extensive fibroblastic stromal positivity reported)
**TmPS= Tumor membrane Percent Score-Tumor membrane target expression calculated by summing the percentages of intensities at either 21+, 22+ or 23+, Scores range from 0 to 100,
17View entire presentation