Ocuphire Pharma Results
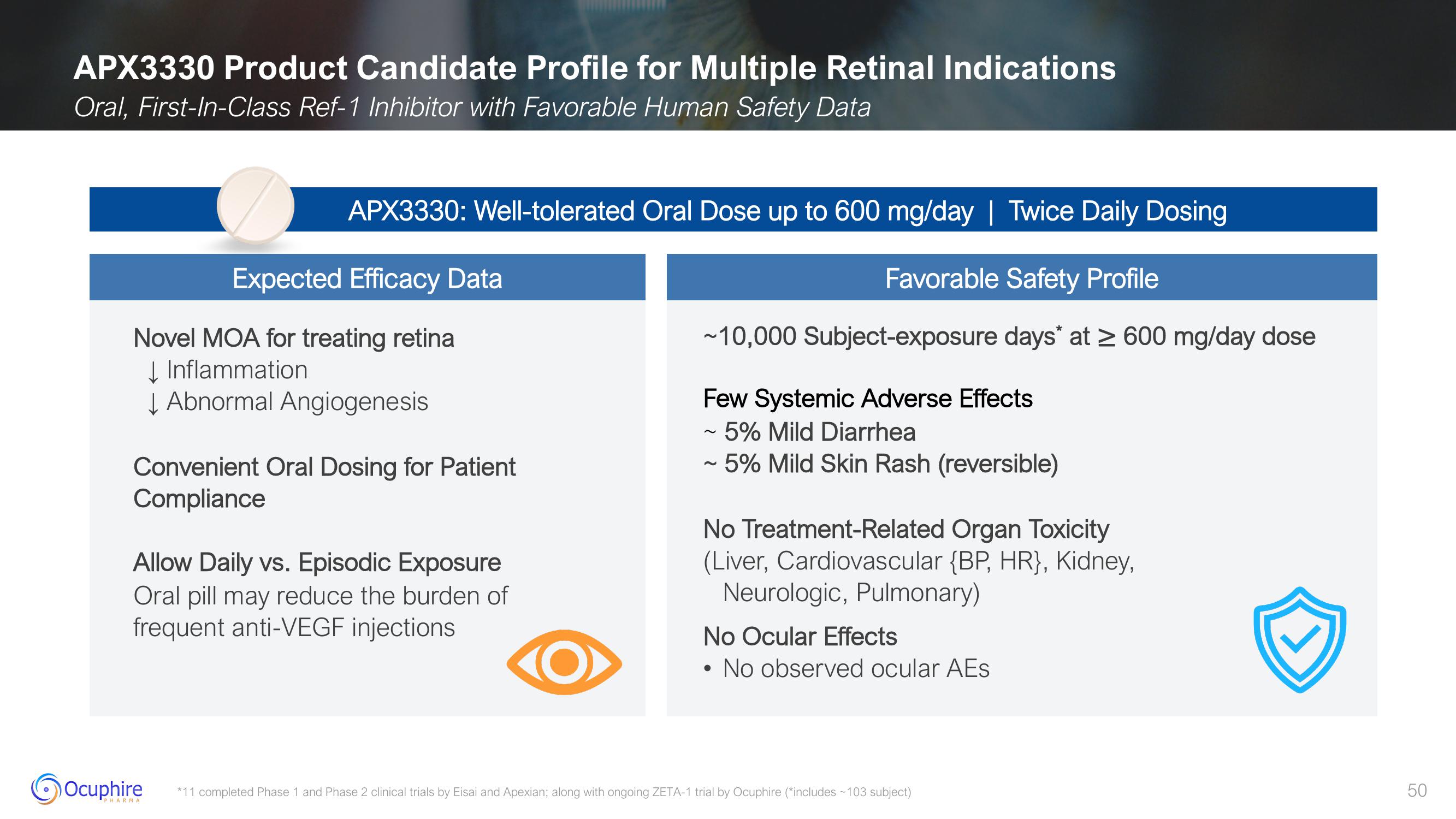
APX3330 Product Candidate Profile for Multiple Retinal Indications
Oral, First-In-Class Ref-1 Inhibitor with Favorable Human Safety Data
APX3330: Well-tolerated Oral Dose up to 600 mg/day | Twice Daily Dosing
Favorable Safety Profile
~10,000 Subject-exposure days* at ≥ 600 mg/day dose
Expected Efficacy Data
Novel MOA for treating retina
↓Inflammation
↓ Abnormal Angiogenesis
Convenient Oral Dosing for Patient
Compliance
Allow Daily vs. Episodic Exposure
Oral pill may reduce the burden of
frequent anti-VEGF injections
O
Few Systemic Adverse Effects
~ 5% Mild Diarrhea
~ 5% Mild Skin Rash (reversible)
No Treatment-Related Organ Toxicity
(Liver, Cardiovascular {BP, HR}, Kidney,
Neurologic, Pulmonary)
No Ocular Effects
No observed ocular AEs
●
Ocuphire *11 completed Phase 1 and Phase 2 clinical trials by Eisai and Apexian; along with ongoing ZETA-1 trial by Ocuphire (*includes ~103 subject)
PHARMA
S
50View entire presentation