AstraZeneca Results Presentation Deck
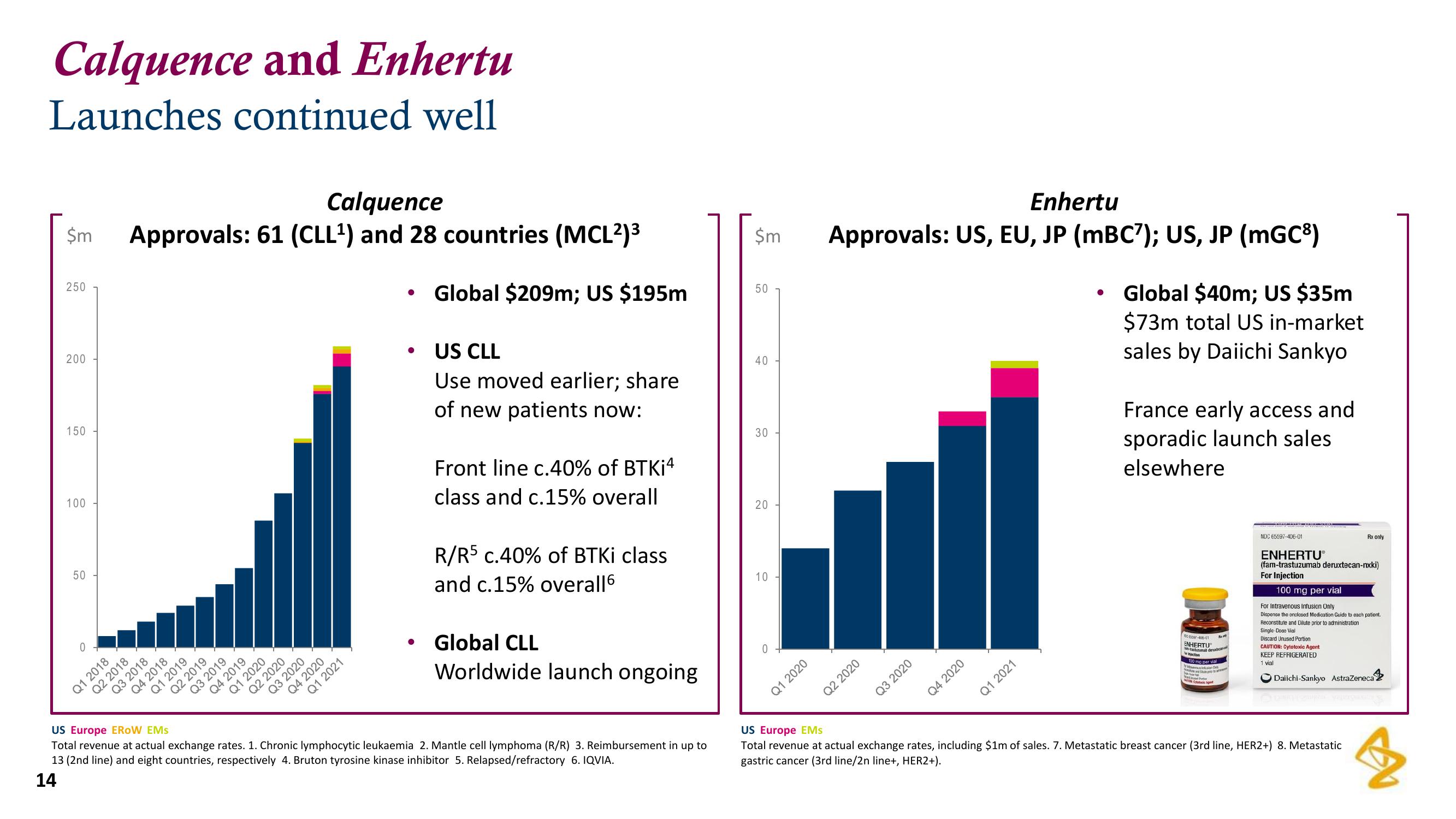
Calquence and Enhertu
Launches continued well
Calquence
$m Approvals: 61 (CLL¹) and 28 countries (MCL²)³
250
200
150
100
50
0
Q1 2018
Q2 2018
Q3 2018
Q4 2018
Q1 2019
Q2 2019
Q3 2019
Q4 2019
Q1 2020
Q2 2020
Q3 2020
Q4 2020
Q1 2021
●
●
●
Global $209m; US $195m
US CLL
Use moved earlier; share
of new patients now:
Front line c.40% of BTKI4
class and c.15% overall
R/R5 c.40% of BTKi class
and c.15% overall6
Global CLL
Worldwide launch ongoing
US Europe EROW EMs
Total revenue at actual exchange rates. 1. Chronic lymphocytic leukaemia 2. Mantle cell lymphoma (R/R) 3. Reimbursement in up to
13 (2nd line) and eight countries, respectively 4. Bruton tyrosine kinase inhibitor 5. Relapsed/refractory 6. IQVIA.
14
$m
50
40
30
20
10
0
Q1 2020
Enhertu
Approvals: US, EU, JP (mBC7); US, JP (mGC³)
Q2 2020
Q3 2020
Q4 2020
Q1 2021
●
Global $40m; US $35m
$73m total US in-market
sales by Daiichi Sankyo
France early access and
sporadic launch sales
elsewhere
00-406-41
ENHERTU
-rastrual dedica
nection
B
100 mg per vial
nu insion Onl
and Dute pro to
Cytotec Age
NDC 65597-406-01
Rx only
ENHERTUⓇ
(fam-trastuzumab deruxtecan-nxki)
For Injection
100 mg per vial
For Intravenous Infusion Only
Dispense the enclosed Medication Guide to each patient.
Reconstitute and Dilute prior to administration
Single-Dose Vial
Discard Unused Portion
CAUTION: Cytotoxic Agent
KEEP REFRIGERATED
1 vial
Daiichi-Sankyo AstraZeneca
US Europe EMS
Total revenue at actual exchange rates, including $1m of sales. 7. Metastatic breast cancer (3rd line, HER2+) 8. Metastatic
gastric cancer (3rd line/2n line+, HER2+).
4View entire presentation