JP Morgan Healthcare Conference Innovation Update
VISION CARE INNOVATION
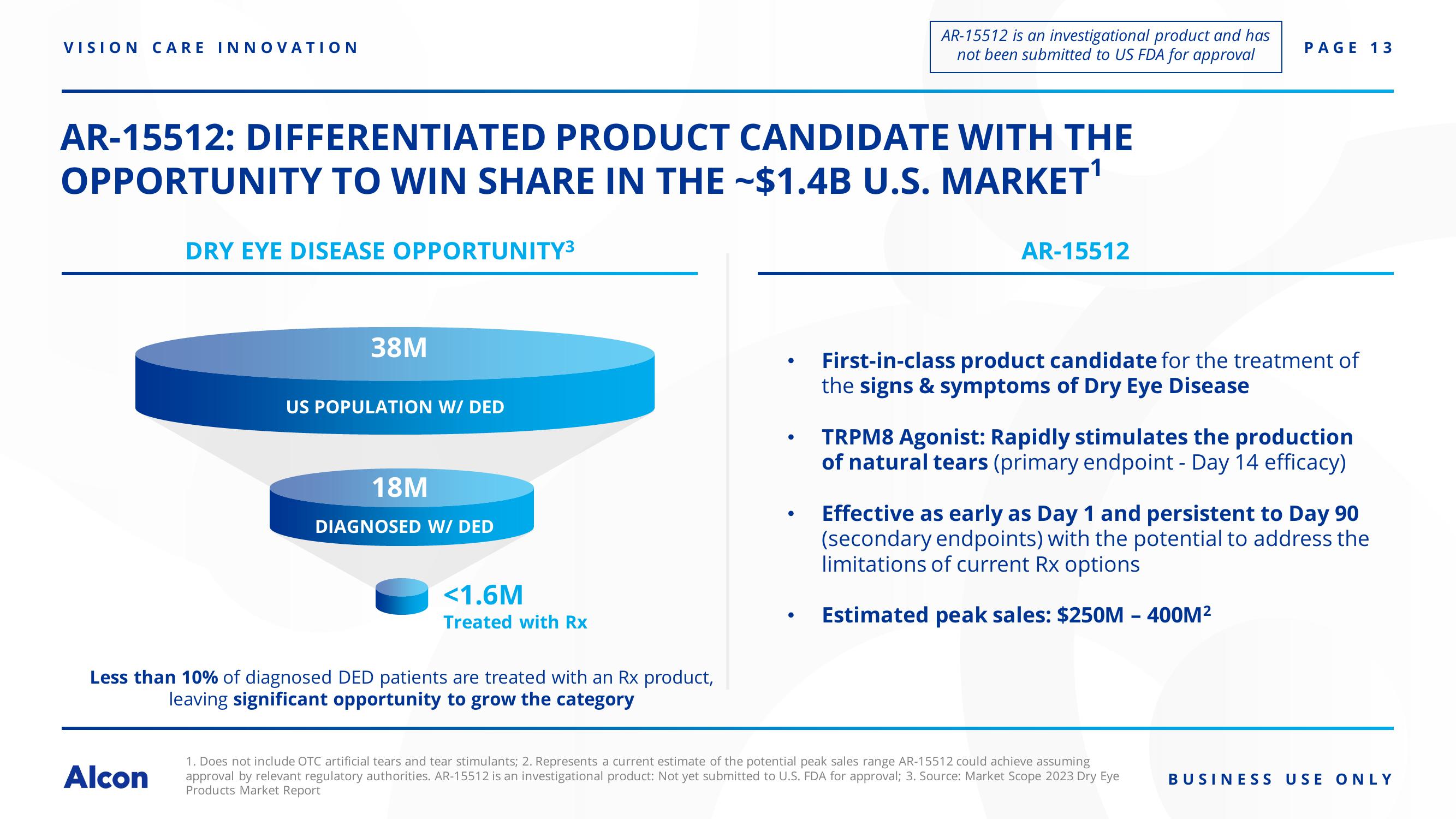
AR-15512: DIFFERENTIATED PRODUCT CANDIDATE WITH THE
OPPORTUNITY TO WIN SHARE IN THE ~$1.4B U.S. MARKET¹
DRY EYE DISEASE OPPORTUNITY³3
Alcon
38M
US POPULATION W/ DED
18M
DIAGNOSED W/ DED
<1.6M
Treated with Rx
Less than 10% of diagnosed DED patients are treated with an Rx product,
leaving significant opportunity to grow the category
●
●
AR-15512 is an investigational product and has
not been submitted to US FDA for approval
●
AR-15512
PAGE 13
First-in-class product candidate for the treatment of
the signs & symptoms of Dry Eye Disease
TRPM8 Agonist: Rapidly stimulates the production
of natural tears (primary endpoint - Day 14 efficacy)
Effective as early as Day 1 and persistent to Day 90
(secondary endpoints) with the potential to address the
limitations of current Rx options
Estimated peak sales: $250M - 400M²
1. Does not include OTC artificial tears and tear stimulants; 2. Represents a current estimate of the potential peak sales range AR-15512 could achieve assuming
approval by relevant regulatory authorities. AR-15512 is an investigational product: Not yet submitted to U.S. FDA for approval; 3. Source: Market Scope 2023 Dry Eye
Products Market Report
BUSINESS USE ONLYView entire presentation