Aeglea BioTherapeutics Investor Presentation Deck
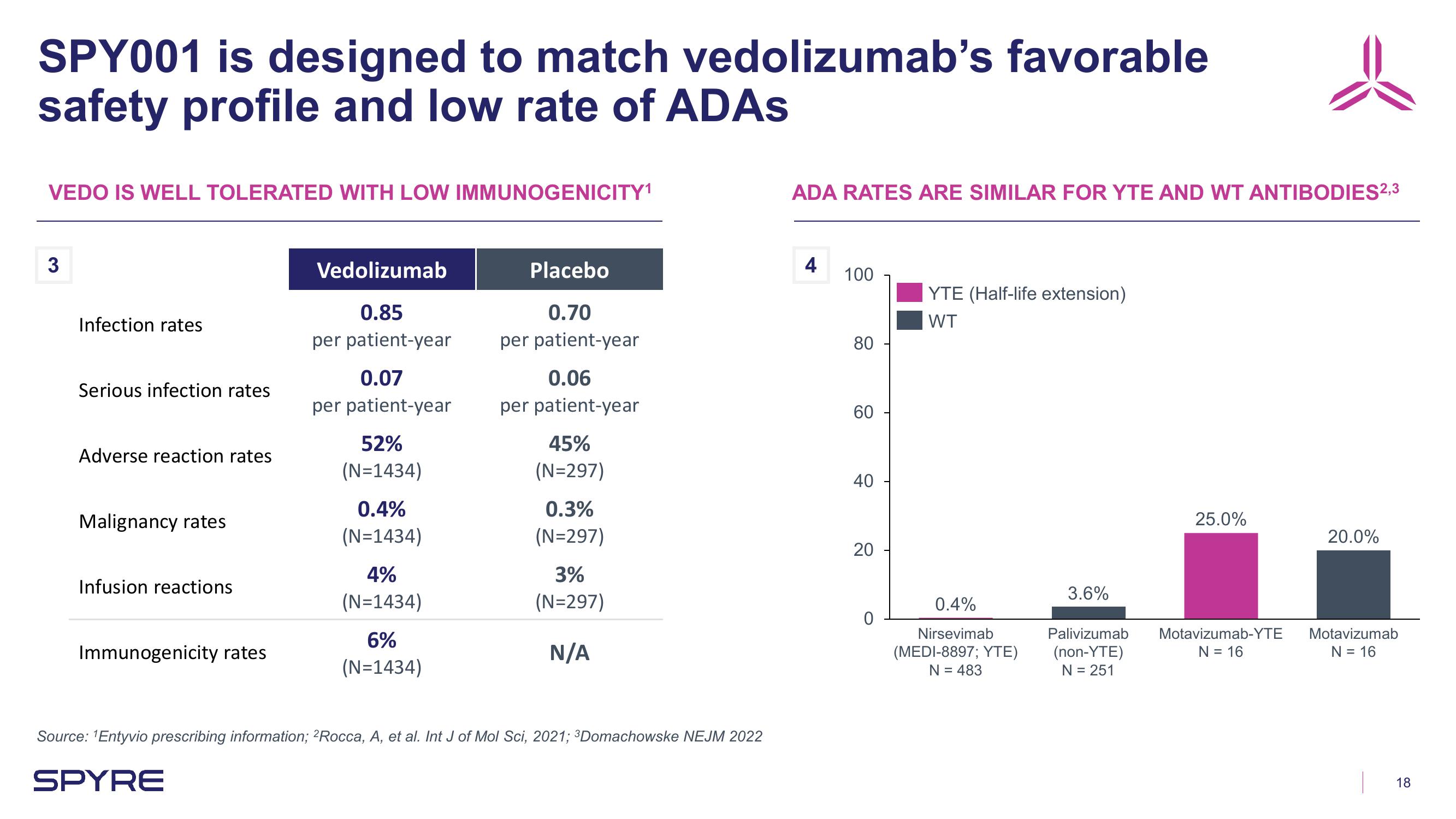
SPY001 is designed to match vedolizumab's favorable
safety profile and low rate of ADAs
VEDO IS WELL TOLERATED WITH LOW IMMUNOGENICITY¹
3
Infection rates
Serious infection rates
Adverse reaction rates
Malignancy rates
Infusion reactions
Immunogenicity rates
Vedolizumab
0.85
per patient-year
0.07
per patient-year
SPYRE
52%
(N=1434)
0.4%
(N=1434)
4%
(N=1434)
6%
(N=1434)
Placebo
0.70
per patient-year
0.06
per patient-year
45%
(N=297)
0.3%
(N=297)
3%
(N=297)
N/A
Source: ¹Entyvio prescribing information; 2Rocca, A, et al. Int J of Mol Sci, 2021; ³Domachowske NEJM 2022
ADA RATES ARE SIMILAR FOR YTE AND WT ANTIBODIES2,3
4
100
80
60
40
20
0
YTE (Half-life extension)
WT
0.4%
Nirsevimab
(MEDI-8897; YTE)
N = 483
3.6%
Palivizumab
(non-YTE)
N = 251
25.0%
Motavizumab-YTE
N = 16
20.0%
Motavizumab
N = 16
18View entire presentation