BioAtla IPO Presentation Deck
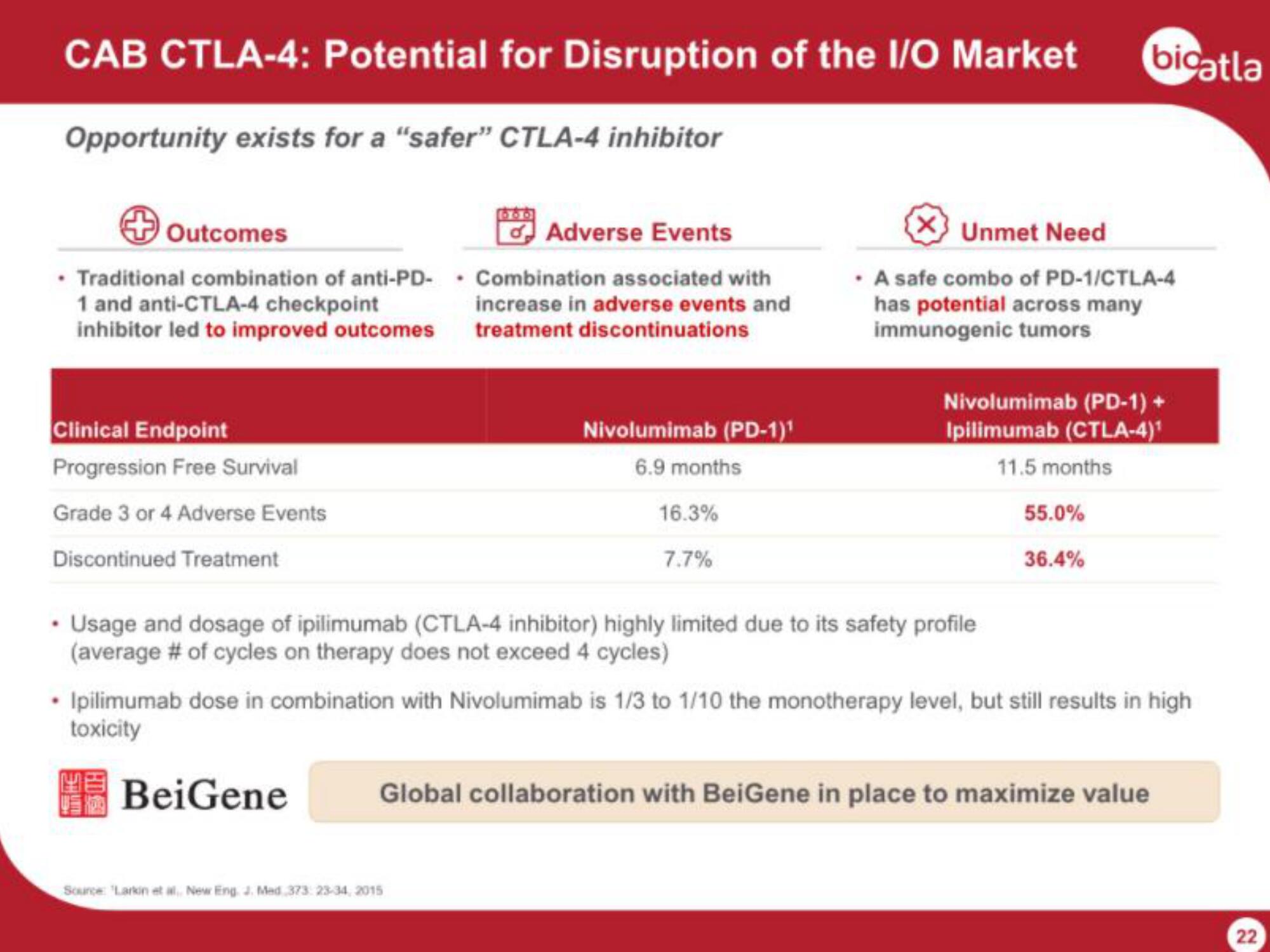
CAB CTLA-4: Potential for Disruption of the I/O Market
Opportunity exists for a "safer" CTLA-4 inhibitor
Outcomes
• Traditional combination of anti-PD-
1 and anti-CTLA-4 checkpoint
inhibitor led to improved outcomes
Clinical Endpoint
Progression Free Survival
Grade 3 or 4 Adverse Events
Discontinued Treatment
Adverse Events
Combination associated with
increase in adverse events and
treatment discontinuations
Nivolumimab (PD-1)¹
6.9 months
16.3%
7.7%
Source: Larkin et al., New Eng: J. Med 373:23-34, 2015
X Unmet Need
• A safe combo of PD-1/CTLA-4
has potential across many
immunogenic tumors
• Usage and dosage of ipilimumab (CTLA-4 inhibitor) highly limited due to its safety profile
(average # of cycles on therapy does not exceed 4 cycles)
bicatla
Nivolumimab (PD-1) +
Ipilimumab (CTLA-4)¹
11.5 months
55.0%
36.4%
• Ipilimumab dose in combination with Nivolumimab is 1/3 to 1/10 the monotherapy level, but still results in high
toxicity
BeiGene
Global collaboration with BeiGene in place to maximize value
22View entire presentation