BioNTech Results Presentation Deck
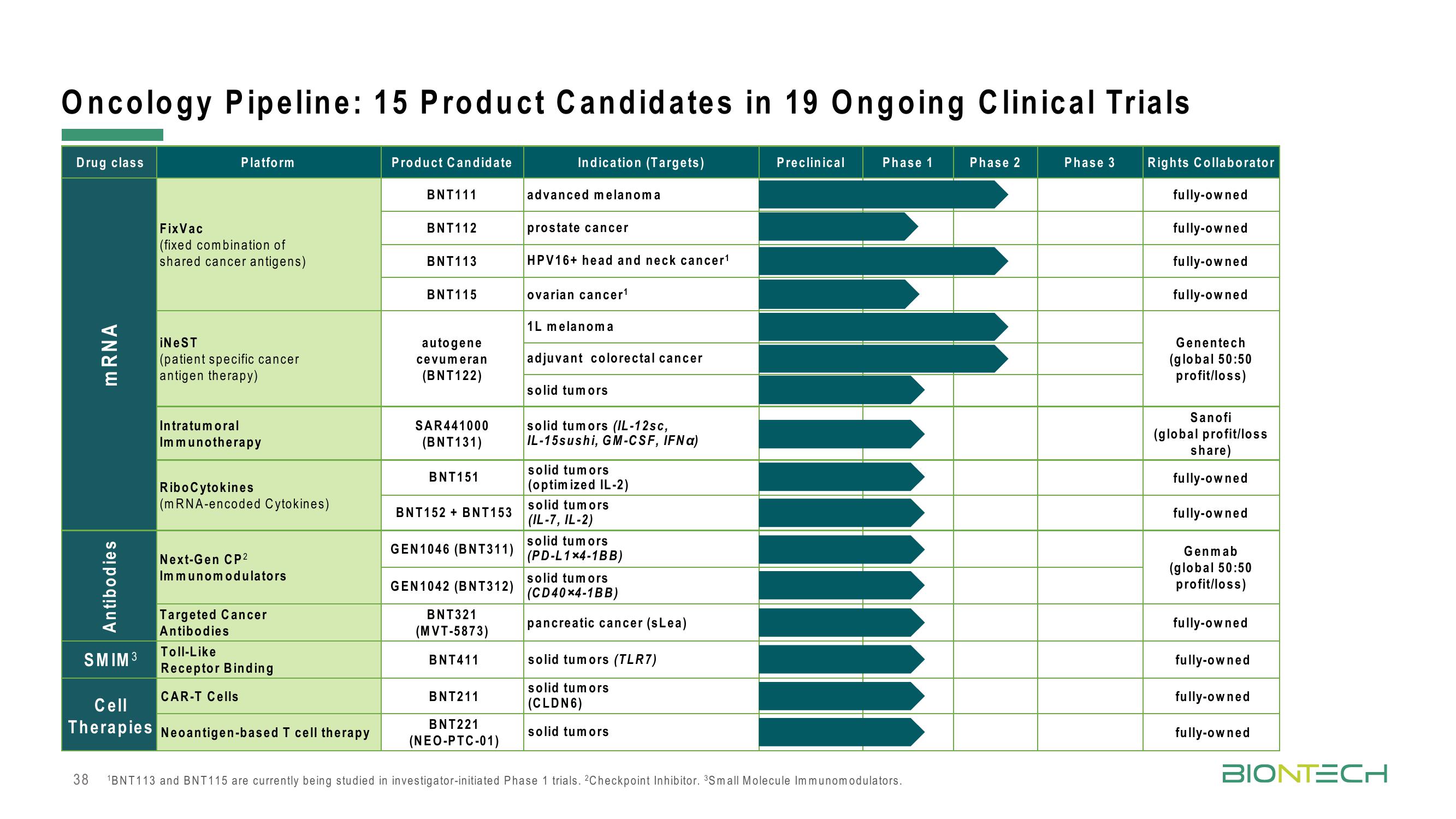
Oncology Pipeline: 15 Product Candidates in 19 Ongoing Clinical Trials
Drug class
mRNA
Antibodies
SMIM ³
38
Platform
Fix Vac
(fixed combination of
shared cancer antigens)
iNeST
(patient specific cancer
antigen therapy)
Intratumoral
Immunotherapy
RiboCytokines
(mRNA-encoded Cytokines)
Next-Gen CP²
Immunomodulators
Targeted Cancer
Antibodies
Toll-Like
Receptor Binding
CAR-T Cells
Cell
Therapies Neoantigen-based T cell therapy
Product Candidate
BNT111
BNT112
BNT113
BNT115
autogene
cevumeran
(BNT122)
SAR441000
(BNT131)
BNT151
BNT152 + BNT153
GEN1046 (BNT311)
GEN1042 (BNT312)
BNT321
(MVT-5873)
BNT411
BNT211
BNT221
(NEO-PTC-01)
Indication (Targets)
advanced melanoma
prostate cancer
HPV16+ head and neck cancer¹
ovarian cancer¹
1L melanoma
adjuvant colorectal cancer
solid tumors
solid tumors (IL-12sc,
IL-15sushi, GM-CSF, IFN a)
solid tumors
(optimized IL-2)
solid tumors
(IL-7, IL-2)
solid tumors
(PD-L1x4-1BB)
solid tumors
(CD40x4-1BB)
pancreatic cancer (sLea)
solid tumors (TLR7)
solid tumors
(CLDN6)
solid tumors
Preclinical
Phase 1
¹BNT113 and BNT115 are currently being studied in investigator-initiated Phase 1 trials. 2Checkpoint Inhibitor. ³Small Molecule Immunomodulators.
Phase 2
Phase 3
Rights Collaborator
fully-owned
fully-owned
fully-owned
fully-owned
Genentech
(global 50:50
profit/loss)
Sanofi
(global profit/loss
share)
fully-owned
fully-owned
Genmab
(global 50:50
profit/loss)
fully-owned
fully-owned
fully-owned
fully-owned
BIONTECHView entire presentation