Ocuphire Pharma Investor Presentation Deck
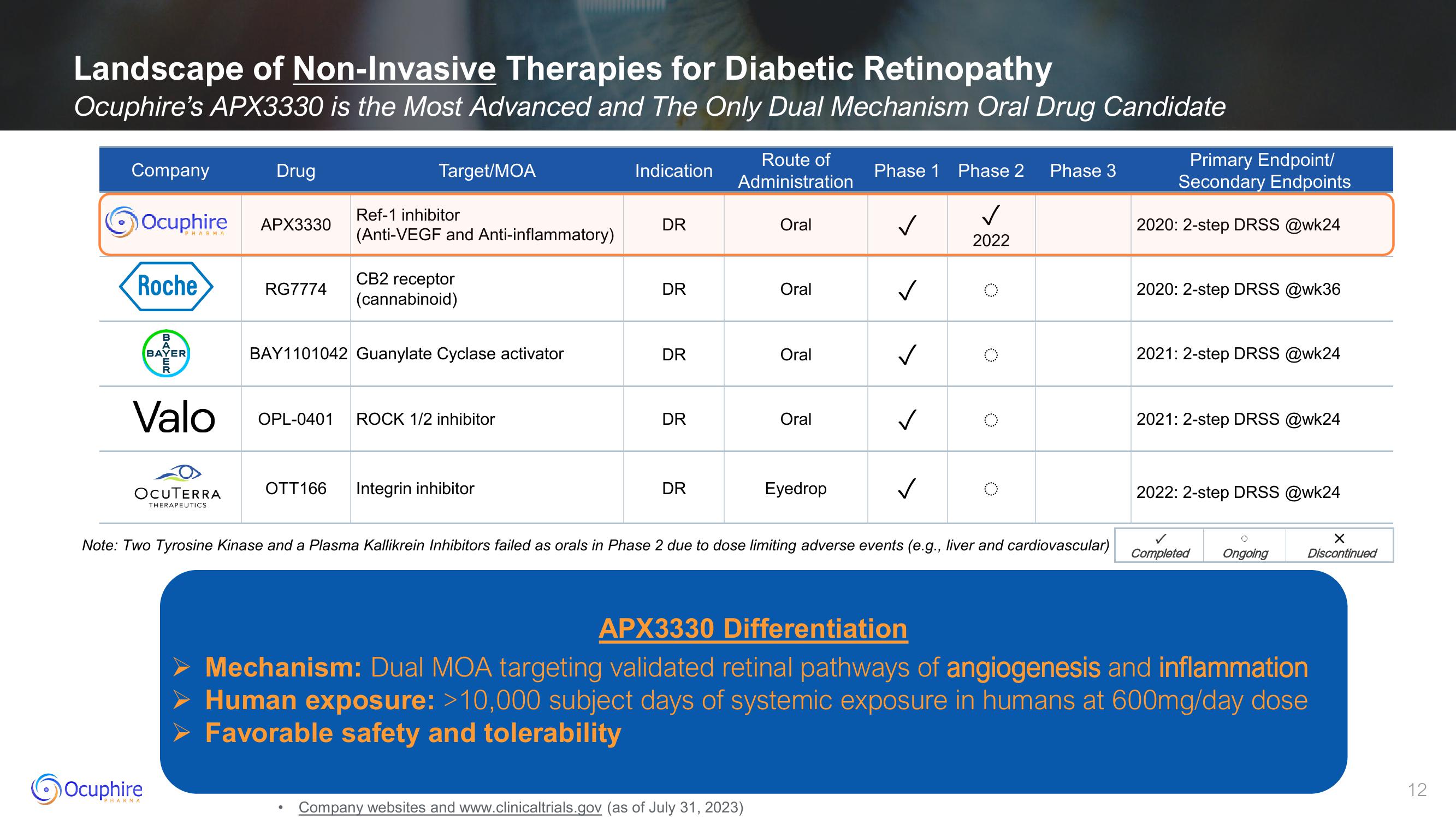
Landscape of Non-Invasive Therapies for Diabetic Retinopathy
Ocuphire's APX3330 is the Most Advanced and The Only Dual Mechanism Oral Drug Candidate
Company
Ocuphire
Roche
PHARMA
BAYER
BAYER
Valo
Ocuphire
OCUTERRA
THERAPEUTICS
Drug
APX3330
RG7774
Target/MOA
Ref-1 inhibitor
(Anti-VEGF and Anti-inflammatory)
CB2 receptor
(cannabinoid)
BAY1101042 Guanylate Cyclase activator
OPL-0401 ROCK 1/2 inhibitor
OTT 166 Integrin inhibitor
Indication
DR
DR
DR
DR
DR
Route of
Administration
Oral
Oral
Company websites and www.clinical trials.gov (as of July 31, 2023)
Oral
Oral
Eyedrop
Phase 1 Phase 2 Phase 3
✓
2022
✓
✓
✓
✓
✓
Primary Endpoint/
Secondary Endpoints
2020: 2-step DRSS @wk24
2020: 2-step DRSS @wk36
2021: 2-step DRSS @wk24
2021: 2-step DRSS @wk24
Note: Two Tyrosine Kinase and a Plasma Kallikrein Inhibitors failed as orals in Phase 2 due to dose limiting adverse events (e.g., liver and cardiovascular) Completed Ongoing
2022: 2-step DRSS @wk24
APX3330 Differentiation
➤ Mechanism: Dual MOA targeting validated retinal pathways of angiogenesis and inflammation
Human exposure: >10,000 subject days of systemic exposure in humans at 600mg/day dose
Favorable safety and tolerability
X
Discontinued
12View entire presentation