Taysha IPO Presentation Deck
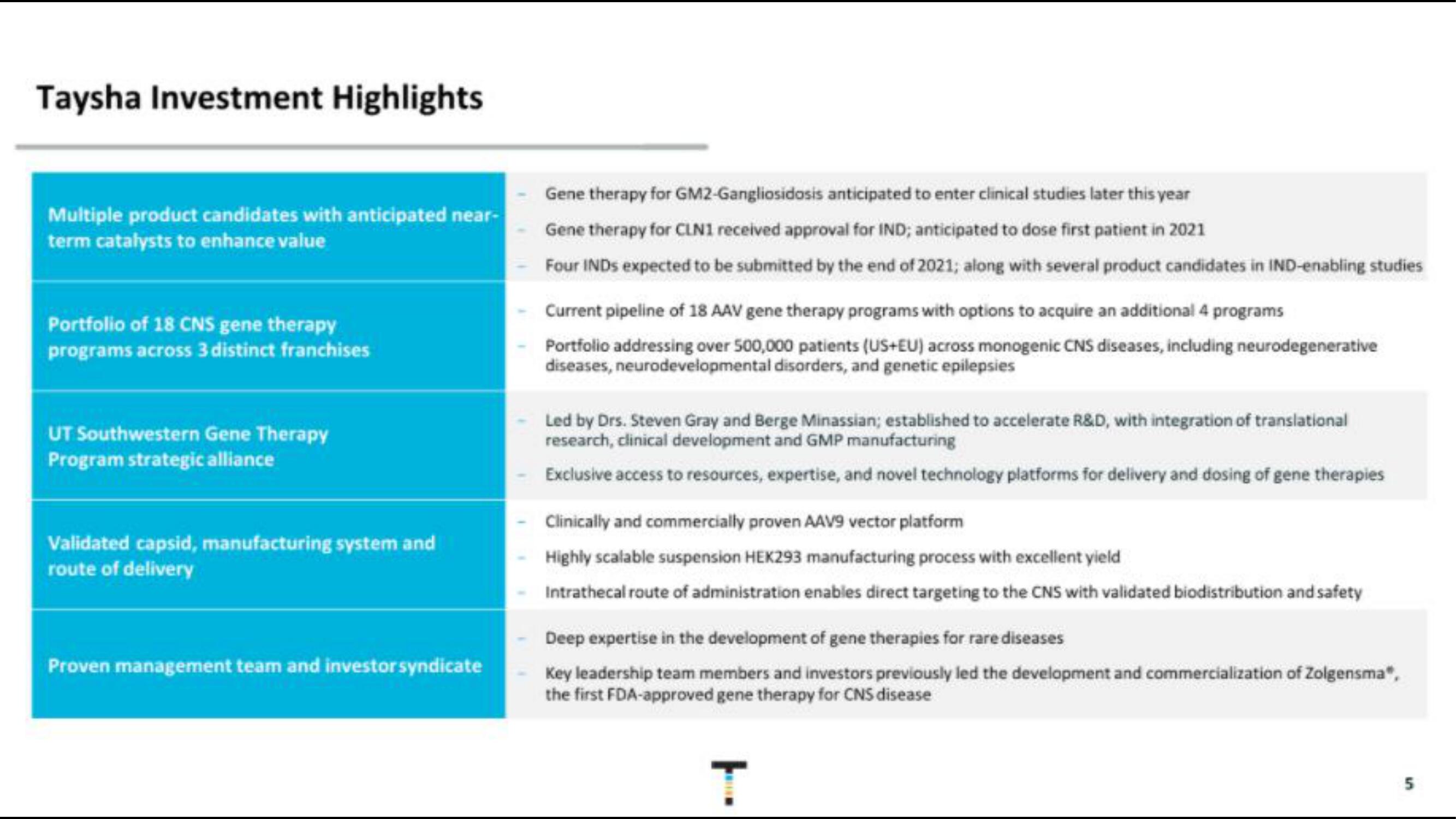
Taysha Investment Highlights
Multiple product candidates with anticipated near-
term catalysts to enhance value
Portfolio of 18 CNS gene therapy
programs across 3 distinct franchises
UT Southwestern Gene Therapy
Program strategic alliance
Validated capsid, manufacturing system and
route of delivery
Proven management team and investor syndicate
Gene therapy for GM2-Gangliosidosis anticipated to enter clinical studies later this year
Gene therapy for CLN1 received approval for IND; anticipated to dose first patient in 2021
Four INDS expected to be submitted by the end of 2021; along with several product candidates in IND-enabling studies
Current pipeline of 18 AAV gene therapy programs with options to acquire an additional 4 programs
Portfolio addressing over 500,000 patients (US+EU) across monogenic CNS diseases, including neurodegenerative
diseases, neurodevelopmental disorders, and genetic epilepsies
Led by Drs. Steven Gray and Berge Minassian; established to accelerate R&D, with integration of translational
research, clinical development and GMP manufacturing
Exclusive access to resources, expertise, and novel technology platforms for delivery and dosing of gene therapies
Clinically and commercially proven AAV9 vector platform
Highly scalable suspension HEK293 manufacturing process with excellent yield
Intrathecal route of administration enables direct targeting to the CNS with validated biodistribution and safety
Deep expertise in the development of gene therapies for rare diseases
Key leadership team members and investors previously led the development and commercialization of Zolgensma",
the first FDA-approved gene therapy for CNS disease
5View entire presentation