Certara Investor Day Presentation Deck
2021 novel drug approval using Simcyp Simulator
Multi-discipline Review for voclosporin
Center for Drug Evaluation and Research
All simulations were performed using the PK/PD Profiles mode in the Simcyp®
Simulator (Version 17 Certara, Sheffield, UK). A scheme of the PBPK simulation
strategy is shown in Figure 21, which summarizes the studies used for model
development and verification, and model applications in DDI predictions.
Simcyp library files of ketoconazole, diltiazem, verapamil and nor-
verapamil, fluconazole, fluvoxamine, cimetidine, rifampin MD, efavirenz,
rosuvastatin and pravastatin were used for DDI simulations without any
modification except that a P-gp Ki of 0.059 M was incorporated into the
sim-ketoconazole 400 mg QD file and ketoconazole CL/F was reduced to
3.7 L/h (see comments in the Results section).
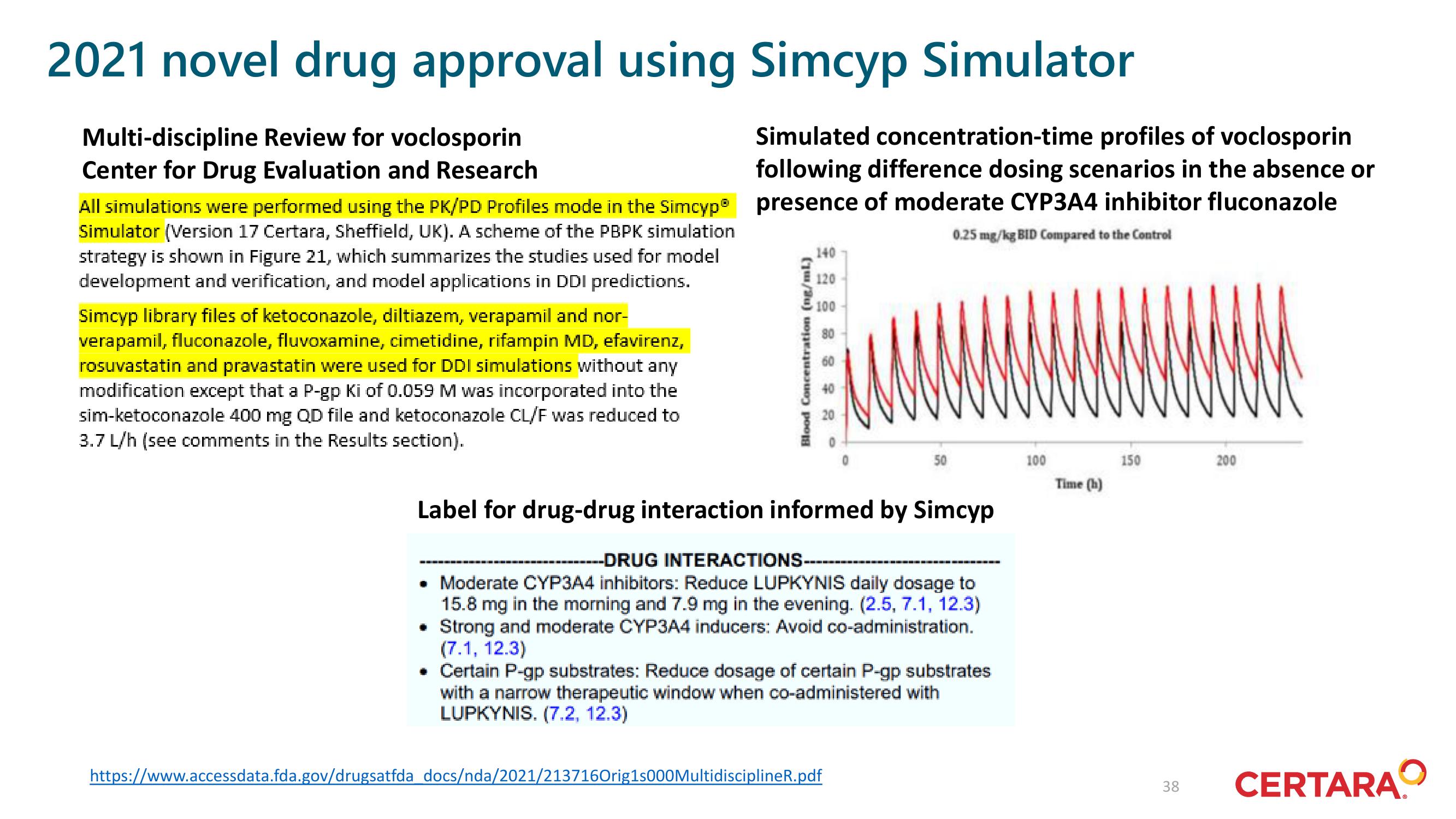
Simulated concentration-time profiles of voclosporin
following difference dosing scenarios in the absence or
presence of moderate CYP3A4 inhibitor fluconazole
0.25 mg/kg BID Compared to the Control
Blood Concentration (ng/mL)
140
120
100
80
60
40
20
50
Label for drug-drug interaction informed by Simcyp
-DRUG INTERACTIONS-
. Moderate CYP3A4 inhibitors: Reduce LUPKYNIS daily dosage to
15.8 mg in the morning and 7.9 mg in the evening. (2.5, 7.1, 12.3)
. Strong and moderate CYP3A4 inducers: Avoid co-administration.
(7.1, 12.3)
+ Certain P-gp substrates: Reduce dosage of certain P-gp substrates
with a narrow therapeutic window when co-administered with
LUPKYNIS. (7.2, 12.3)
https://www.accessdata.fda.gov/drugsatfda_docs/nda/2021/213716Orig1s000MultidisciplineR.pdf
100
Time (h)
150
38
200
CERTARAView entire presentation