MaxCyte Investor Presentation Deck
EXPERT™ Platform Addresses Industry Challenges
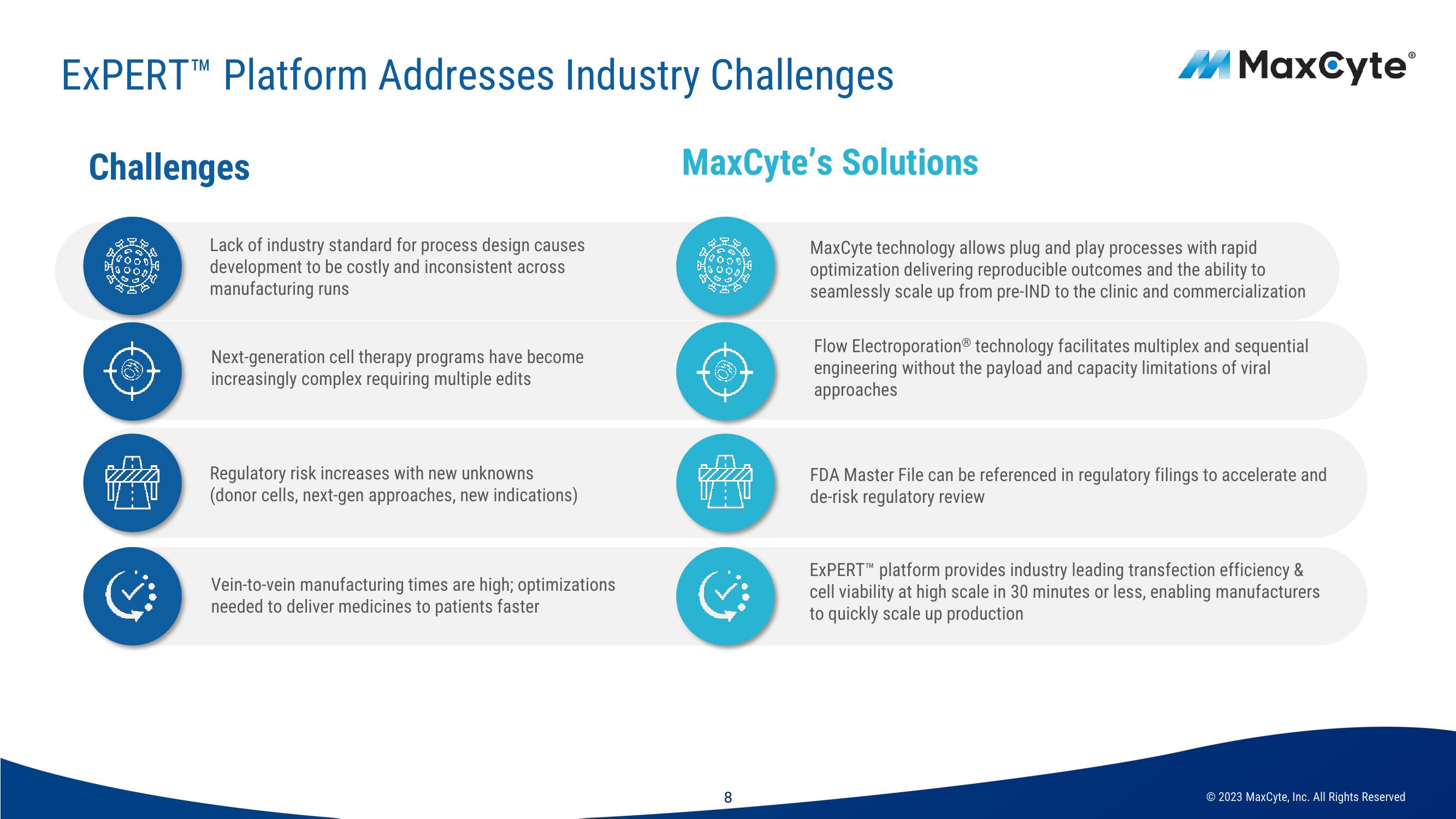
Challenges
A⁰
C
Lack of industry standard for process design causes
development to be costly and inconsistent across
manufacturing runs
Next-generation cell therapy programs have become
increasingly complex requiring multiple edits
Regulatory risk increases with new unknowns
(donor cells, next-gen approaches, new indications)
Vein-to-vein manufacturing times are high; optimizations
needed to deliver medicines to patients faster
MaxCyte's Solutions
A
(✓:
8
M MaxCyte
MaxCyte technology allows plug and play processes with rapid
optimization delivering reproducible outcomes and the ability to
seamlessly scale up from pre-IND to the clinic and commercialization
Flow Electroporation® technology facilitates multiplex and sequential
engineering without the payload and capacity limitations of viral
approaches
FDA Master File can be referenced in regulatory filings to accelerate and
de-risk regulatory review
EXPERT™ platform provides industry leading transfection efficiency &
cell viability at high scale in 30 minutes or less, enabling manufacturers
to quickly scale up production
© 2023 MaxCyte, Inc. All Rights ReservedView entire presentation