Evotec Investor Day Presentation Deck
evotec
PAGE 30
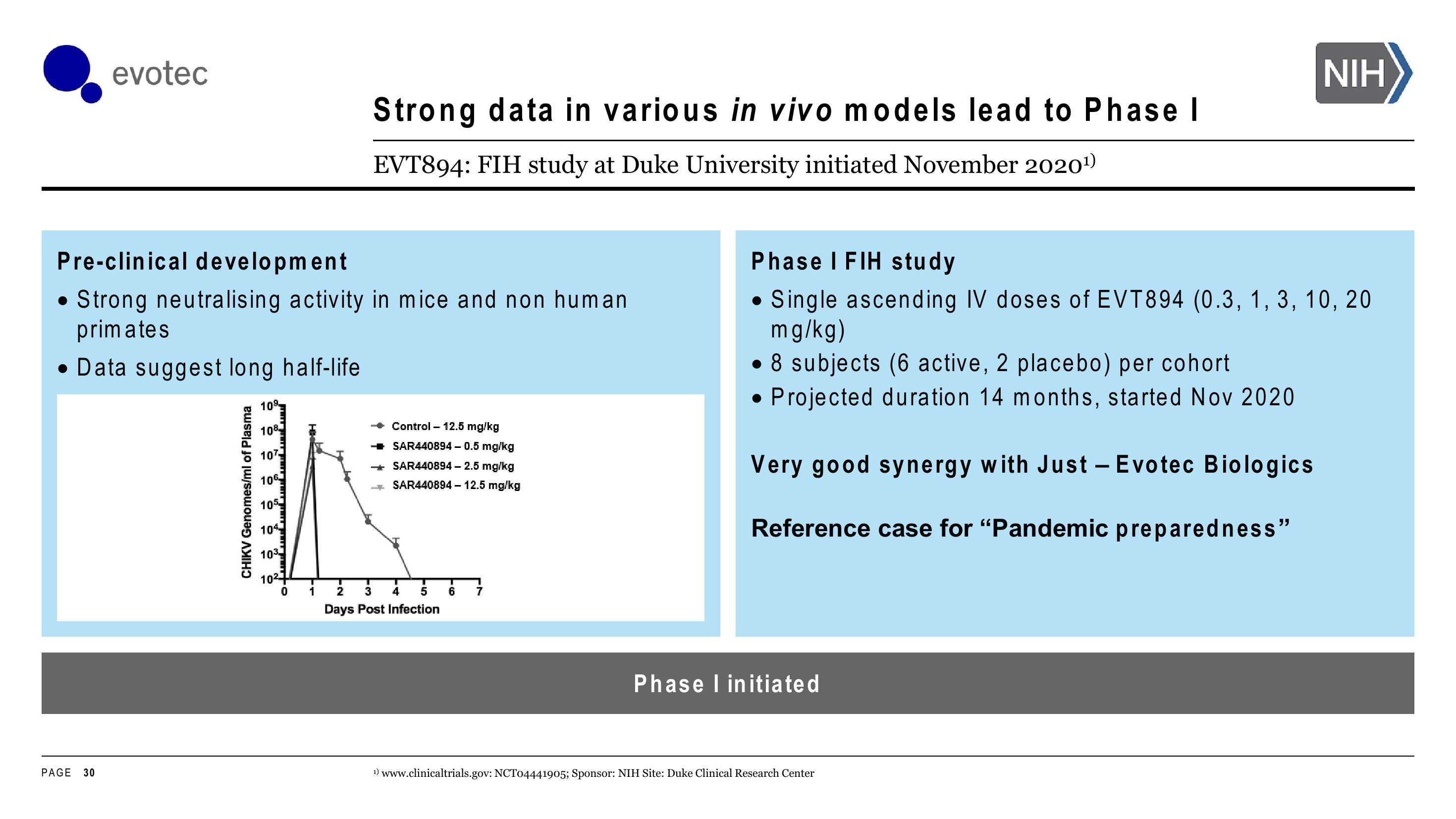
Pre-clinical development
Strong neutralising activity in mice and non human
primates
• Data suggest long half-life
CHIKV Genomes/ml of Plasma
109
108
107
106
105
104
103
10²
0
T
1
Strong data in various in vivo models lead to Phase I
EVT894: FIH study at Duke University initiated November 2020¹)
T
Control - 12.5 mg/kg
SAR440894 - 0.5 mg/kg
SAR440894 - 2.5 mg/kg
SAR440894 - 12.5 mg/kg
2
3 4 5
Days Post Infection
1
Phase I FIH study
Single ascending IV doses of EVT894 (0.3, 1, 3, 10, 20
mg/kg)
• 8 subjects (6 active, 2 placebo) per cohort
Projected duration 14 months, started Nov 2020
Very good synergy with Just - Evotec Biologics
Reference case for "Pandemic preparedness"
Phase I initiated
NIH
1) www.clinicaltrials.gov: NCT04441905; Sponsor: NIH Site: Duke Clinical Research CenterView entire presentation