Imara M&A
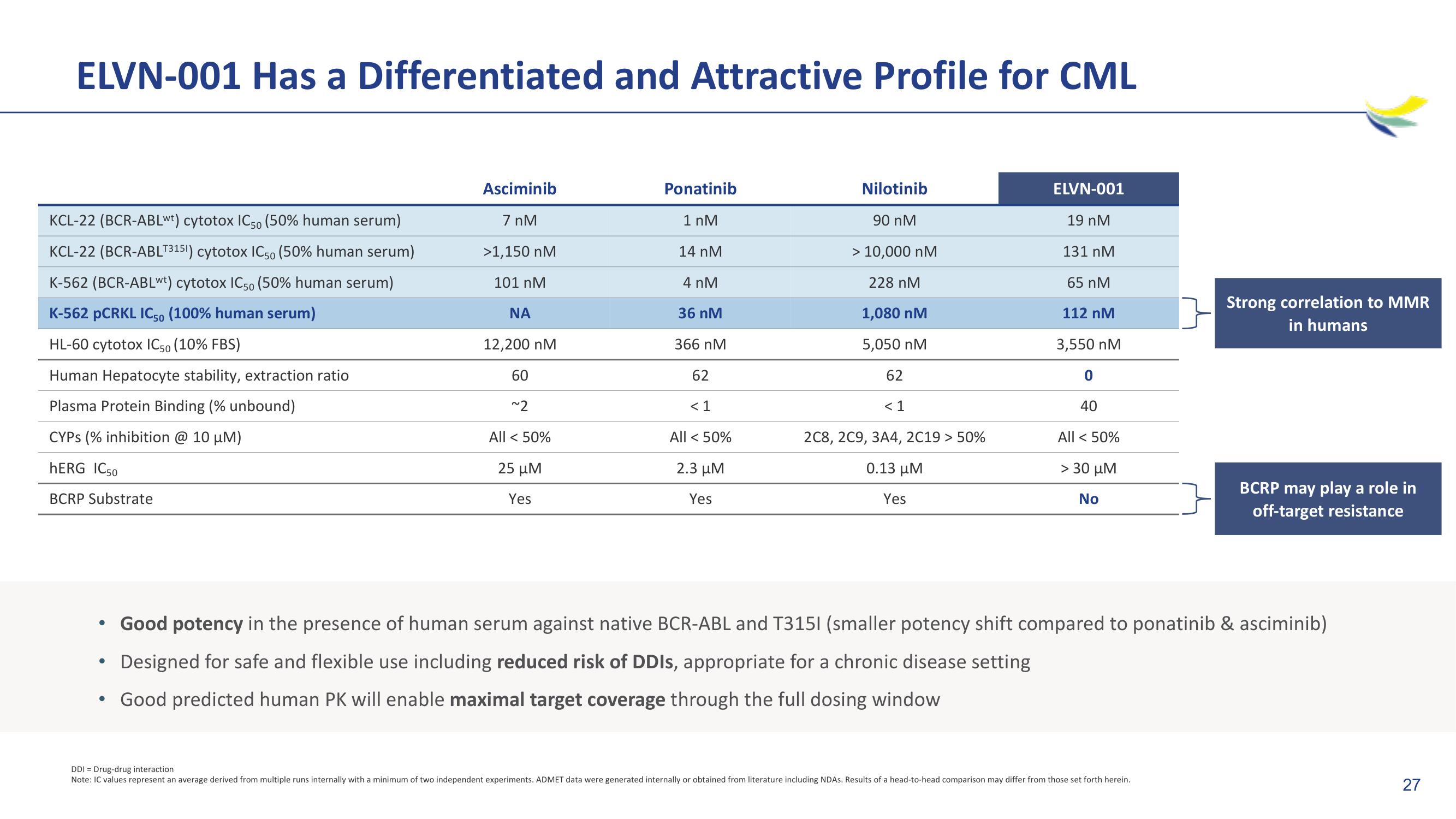
ELVN-001 Has a Differentiated and Attractive Profile for CML
KCL-22 (BCR-ABLwt) cytotox IC50 (50% human serum)
KCL-22 (BCR-ABLT3151) cytotox IC50 (50% human serum)
K-562 (BCR-ABLwt) cytotox IC50 (50% human serum)
K-562 pCRKL IC50 (100% human serum)
HL-60 cytotox IC50 (10% FBS)
Human Hepatocyte stability, extraction ratio
Plasma Protein Binding (% unbound)
CYPS (% inhibition @ 10 μM)
hERG IC50
BCRP Substrate
●
Asciminib
7 nM
>1,150 nM
101 nM
ΝΑ
12,200 nM
●
60
~2
All < 50%
25 μΜ
Yes
Ponatinib
1 nM
14 nM
4 nM
36 nM
366 nM
62
<1
All < 50%
2.3 μM
Yes
Nilotinib
90 nM
> 10,000 nM
228 nM
1,080 nM
5,050 nM
62
< 1
2C8, 2C9, 3A4, 2C19 > 50%
0.13 μΜ
Yes
ELVN-001
19 nM
131 nM
65 nM
112 nM
3,550 nM
0
40
All < 50%
> 30 μη
No
Good potency in the presence of human serum against native BCR-ABL and T3151 (smaller potency shift compared to ponatinib & asciminib)
Designed for safe and flexible use including reduced risk of DDIs, appropriate for a chronic disease setting
• Good predicted human PK will enable maximal target coverage through the full dosing window
Strong correlation to MMR
in humans
DDI = Drug-drug interaction
Note: IC values represent an average derived from multiple runs internally with a minimum of two independent experiments. ADMET data were generated internally or obtained from literature including NDAs. Results of a head-to-head comparison may differ from those set forth herein.
BCRP may play a role in
off-target resistance
27View entire presentation