Kymera Investor Presentation Deck
●
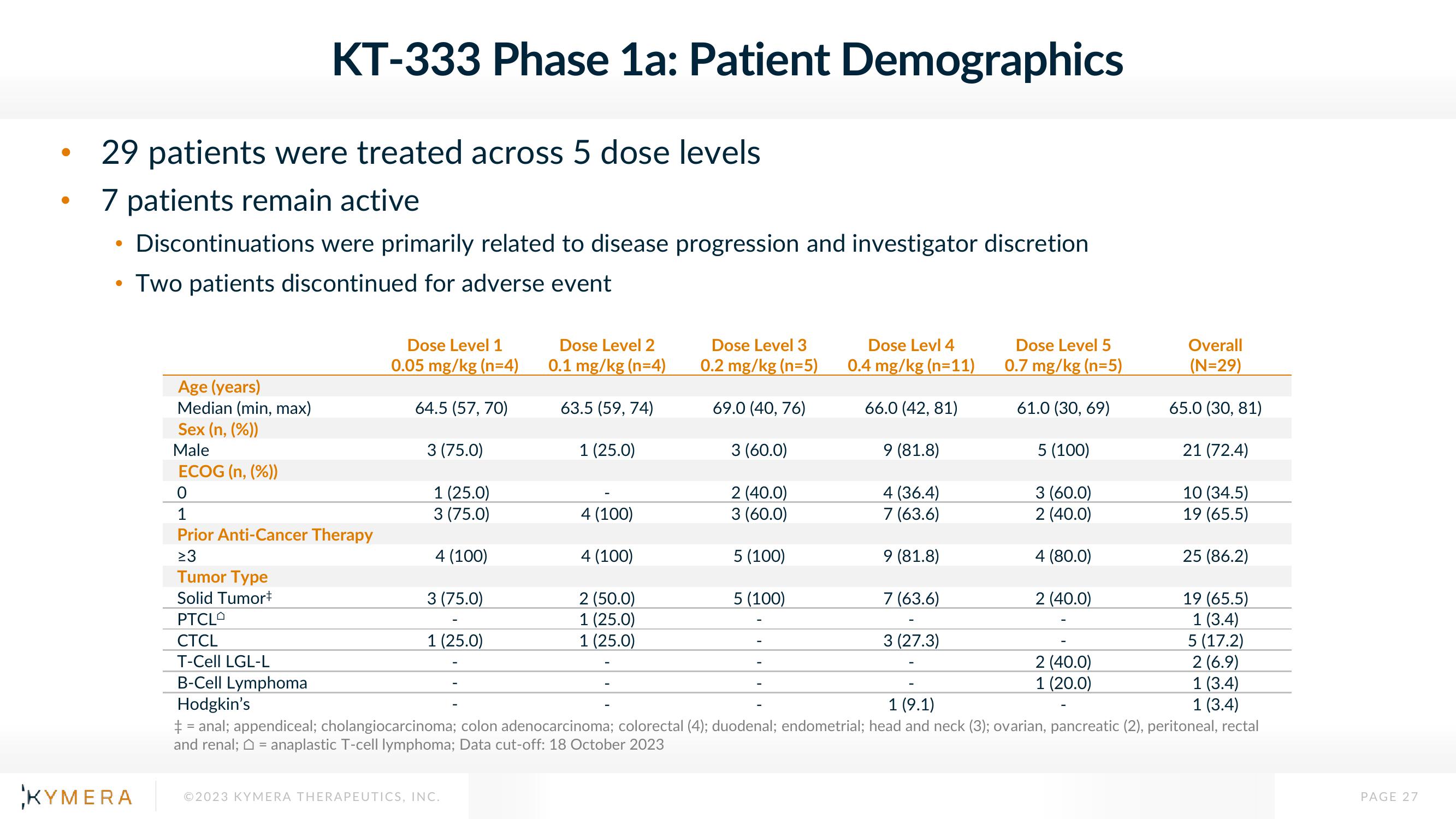
29 patients were treated across 5 dose levels
7 patients remain active
•
KYMERA
Discontinuations were primarily related to disease progression and investigator discretion
Two patients discontinued for adverse event
Age (years)
Median (min, max)
KT-333 Phase 1a: Patient Demographics
Sex (n, (%))
Male
ECOG (n, (%))
0
1
Prior Anti-Cancer Therapy
≥3
Dose Level 1
0.05 mg/kg (n=4)
64.5 (57, 70)
3 (75.0)
1 (25.0)
3 (75.0)
4 (100)
3 (75.0)
1 (25.0)
Dose Level 2
0.1 mg/kg (n=4)
Ⓒ2023 KYMERA THERAPEUTICS, INC.
63.5 (59, 74)
1 (25.0)
4 (100)
4 (100)
Dose Level 3
0.2 mg/kg (n=5)
2 (50.0)
1 (25.0)
1 (25.0)
69.0 (40,76)
3 (60.0)
2 (40.0)
3 (60.0)
5 (100)
Dose Levl 4
0.4 mg/kg (n=11)
66.0 (42, 81)
5 (100)
9 (81.8)
4 (36.4)
7 (63.6)
9 (81.8)
Dose Level 5
0.7 mg/kg (n=5)
61.0 (30, 69)
5 (100)
3 (60.0)
2 (40.0)
7 (63.6)
3 (27.3)
4 (80.0)
Tumor Type
Solid Tumor+
PTCLO
CTCL
T-Cell LGL-L
B-Cell Lymphoma
Hodgkin's
1 (9.1)
+ = anal; appendiceal; cholangiocarcinoma; colon adenocarcinoma; colorectal (4); duodenal; endometrial; head and neck (3); ovarian, pancreatic (2), peritoneal, rectal
and renal; = anaplastic T-cell lymphoma; Data cut-off: 18 October 2023
2 (40.0)
Overall
(N=29)
65.0 (30, 81)
2 (40.0)
1 (20.0)
21 (72.4)
10 (34.5)
19 (65.5)
25 (86.2)
19 (65.5)
1 (3.4)
5 (17.2)
2 (6.9)
1 (3.4)
1 (3.4)
PAGE 27View entire presentation