BenevolentAI Investor Presentation Deck
BEN-8744 - Ulcerative Colitis (UC)
Affects 0.4% US population (¹), 1.7 million
patients in 7MM (2), forecast $7.8bn market by
2026(3)
• A chronic, lifelong disease that causes inflammation and
ulceration of the inner lining of the colon and rectum
• Efficacy - 20-40% of Moderate-severe patients do not respond
to anti-TNF (main treatment paradigm)
• Safety - Treatments have many side effects from steroids to
anti-TNF and JAK inhibitors (black box warnings)
• High unmet need for an alternative oral small molecule
treatment option with improved safety profile and efficacy in
treatment of refractory patients
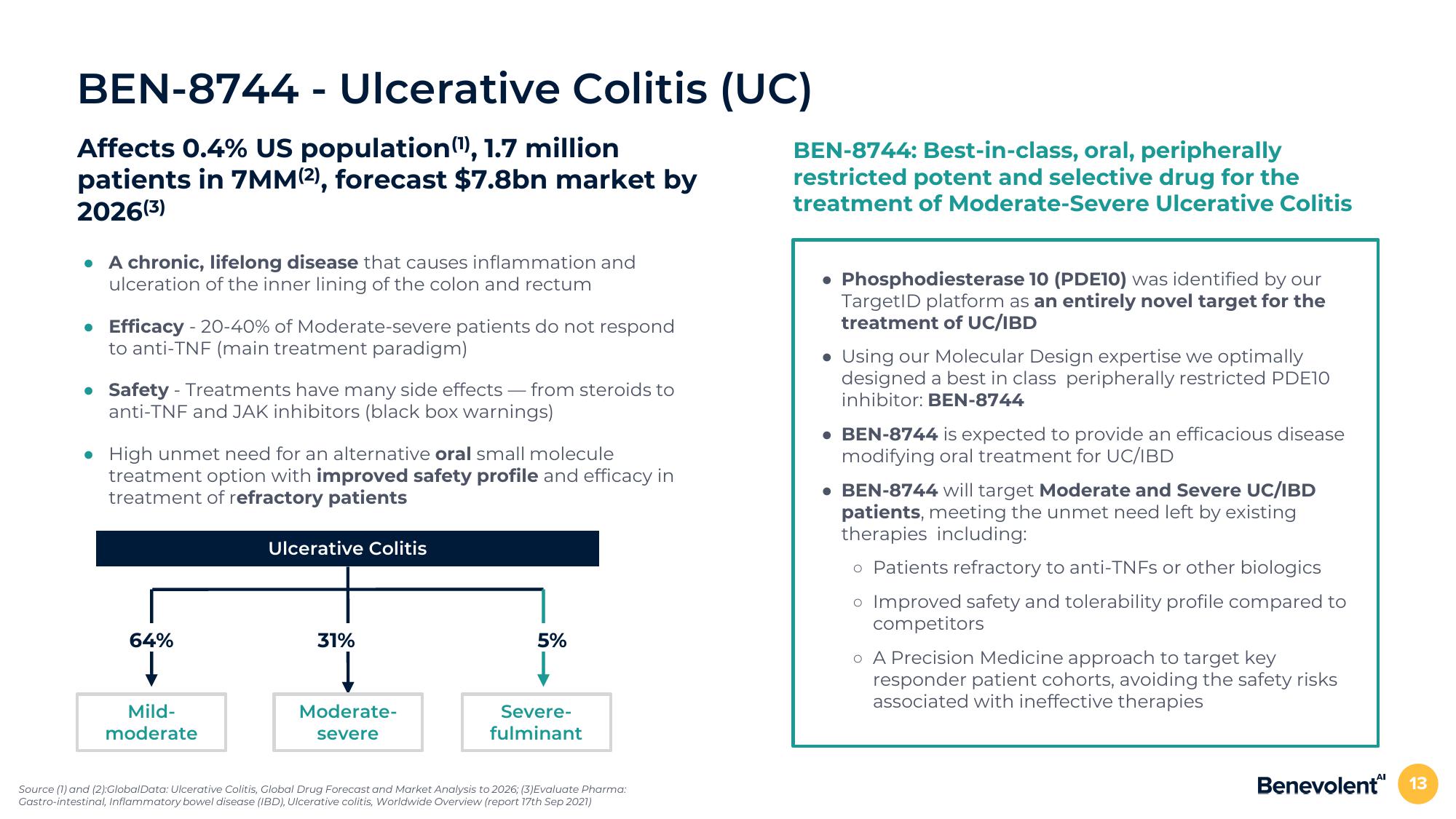
64%
Mild-
moderate
Ulcerative Colitis
31%
Moderate-
severe
5%
Severe-
fulminant
Source (1) and (2):GlobalData: Ulcerative Colitis, Global Drug Forecast and Market Analysis to 2026; (3) Evaluate Pharma:
Gastro-intestinal, Inflammatory bowel disease (IBD), Ulcerative colitis, Worldwide Overview (report 17th Sep 2021)
BEN-8744: Best-in-class, oral, peripherally
restricted potent and selective drug for the
treatment of Moderate-Severe Ulcerative Colitis
• Phosphodiesterase 10 (PDE10) was identified by our
TargetID platform as an entirely novel target for the
treatment of UC/IBD
• Using our Molecular Design expertise we optimally
designed a best in class peripherally restricted PDE10
inhibitor: BEN-8744
• BEN-8744 is expected to provide an efficacious disease
modifying oral treatment for UC/IBD
• BEN-8744 will target Moderate and Severe UC/IBD
patients, meeting the unmet need left by existing
therapies including:
o Patients refractory to anti-TNFS or other biologics
o Improved safety and tolerability profile compared to
competitors
o A Precision Medicine approach to target key
responder patient cohorts, avoiding the safety risks
associated with ineffective therapies
Benevolent 13View entire presentation