Ocuphire Pharma Investor Day Presentation Deck
P
87
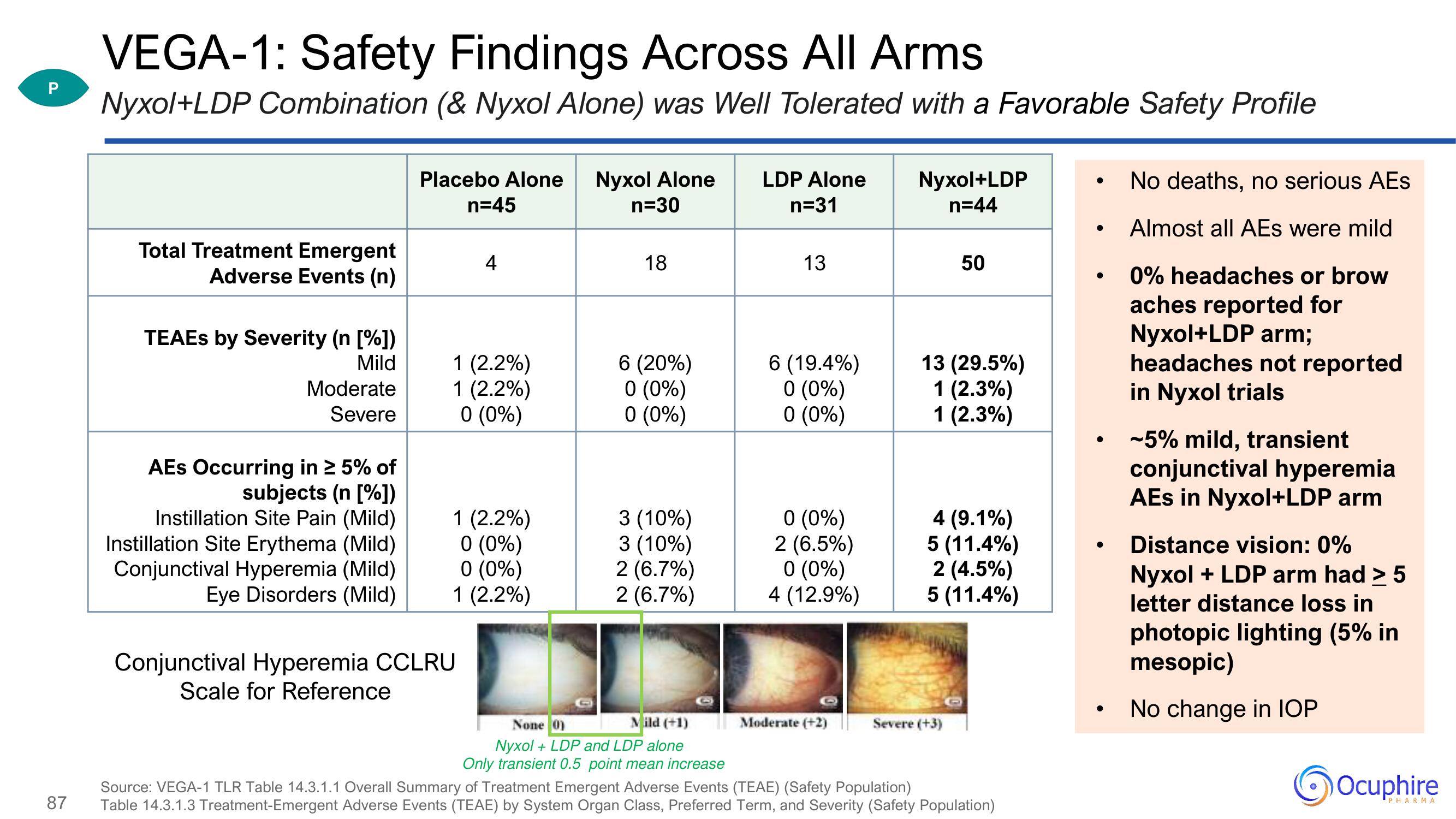
VEGA-1: Safety Findings Across All Arms
Nyxol+LDP Combination (& Nyxol Alone) was Well Tolerated with a Favorable Safety Profile
Total Treatment Emergent
Adverse Events (n)
TEAES by Severity (n [%])
Mild
Moderate
Severe
AEs Occurring in ≥ 5% of
subjects (n [%])
Instillation Site Pain (Mild)
Instillation Site Erythema (Mild)
Conjunctival Hyperemia (Mild)
Eye Disorders (Mild)
Placebo Alone Nyxol Alone
n=45
n=30
4
1 (2.2%)
1 (2.2%)
0 (0%)
1 (2.2%)
0 (0%)
0 (0%)
1 (2.2%)
Conjunctival Hyperemia CCLRU
Scale for Reference
18
6 (20%)
0 (0%)
0 (0%)
3 (10%)
3 (10%)
2 (6.7%)
2 (6.7%)
None 0)
Mild (+1)
Nyxol + LDP and LDP alone
Only transient 0.5 point mean increase
LDP Alone
n=31
13
6 (19.4%)
0 (0%)
0 (0%)
0 (0%)
2 (6.5%)
0 (0%)
4 (12.9%)
Moderate (+2)
Nyxol+LDP
n=44
50
13 (29.5%)
1 (2.3%)
1 (2.3%)
4 (9.1%)
5 (11.4%)
2 (4.5%)
5 (11.4%)
Severe (+3)
Source: VEGA-1 TLR Table 14.3.1.1 Overall Summary of Treatment Emergent Adverse Events (TEAE) (Safety Population)
Table 14.3.1.3 Treatment-Emergent Adverse Events (TEAE) by System Organ Class, Preferred Term, and Severity (Safety Population)
●
●
●
No deaths, no serious AEs
Almost all AEs were mild
0% headaches or brow
aches reported for
Nyxol+LDP arm;
headaches not reported
in Nyxol trials
~5% mild, transient
conjunctival hyperemia
AEs in Nyxol+LDP arm
Distance vision: 0%
Nyxol + LDP arm had > 5
letter distance loss in
photopic lighting (5% in
mesopic)
No change in IOP
Ocuphire
PHARMAView entire presentation