Kymera Investor Presentation Deck
●
●
●
●
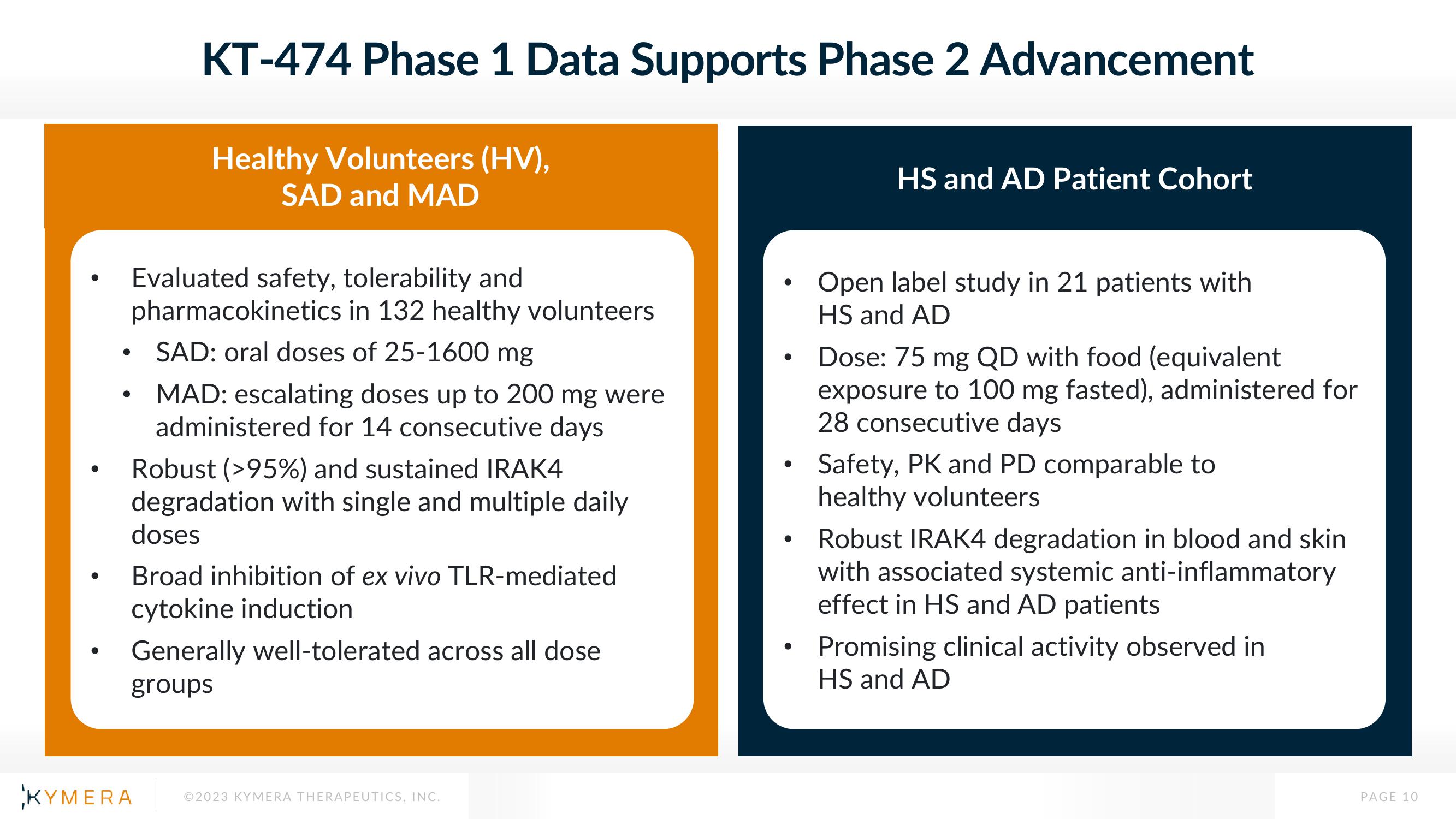
KT-474 Phase 1 Data Supports Phase 2 Advancement
Healthy Volunteers (HV),
SAD and MAD
Evaluated safety, tolerability and
pharmacokinetics in 132 healthy volunteers
SAD: oral doses of 25-1600 mg
MAD: escalating doses up to 200 mg were
administered for 14 consecutive days
Robust ( >95%) and sustained IRAK4
degradation with single and multiple daily
doses
Broad inhibition of ex vivo TLR-mediated
cytokine induction
Generally well-tolerated across all dose
groups
KYMERA
©2023 KYMERA THERAPEUTICS, INC.
●
●
●
●
HS and AD Patient Cohort
Open label study in 21 patients with
HS and AD
Dose: 75 mg QD with food (equivalent
exposure to 100 mg fasted), administered for
28 consecutive days
Safety, PK and PD comparable to
healthy volunteers
Robust IRAK4 degradation in blood and skin
with associated systemic anti-inflammatory
effect in HS and AD patients
Promising clinical activity observed in
HS and AD
PAGE 10View entire presentation