Immix Biopharma Investor Presentation Deck
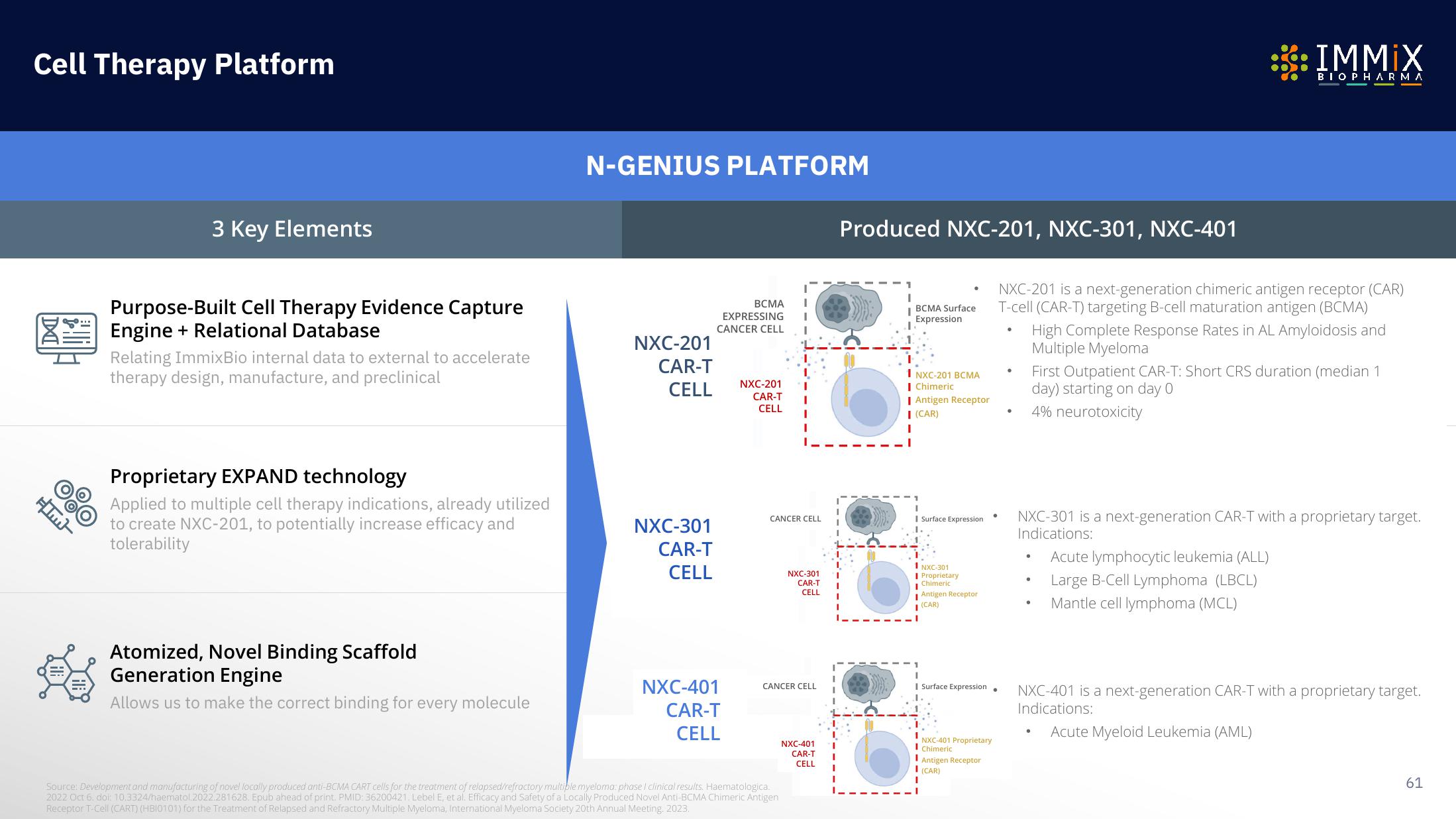
Cell Therapy Platform
HIM
3 Key Elements
Purpose-Built Cell Therapy Evidence Capture
Engine + Relational Database
Relating ImmixBio internal data to external to accelerate
therapy design, manufacture, and preclinical
Proprietary EXPAND technology
Applied to multiple cell therapy indications, already utilized
to create NXC-201, to potentially increase efficacy and
tolerability
Atomized, Novel Binding Scaffold
Generation Engine
Allows us to make the correct binding for every molecule
N-GENIUS PLATFORM
NXC-201
CAR-T
CELL
NXC-301
CAR-T
CELL
BCMA
EXPRESSING
CANCER CELL
NXC-401
CAR-T
CELL
NXC-201
CAR-T
CELL
CANCER CELL
NXC-301
CAR-T
CELL
CANCER CELL
Source: Development and manufacturing of novel locally produced anti-BCMA CART cells for the treatment of relapsed/refractory multiple myeloma: phase I clinical results. Haematologica.
2022 Oct 6. doi: 10.3324/haematol.2022.281628. Epub ahead of print. PMID: 36200421. Lebel E, et al. Efficacy and Safety of a Locally Produced Novel Anti-BCMA Chimeric Antigen
Receptor T-Cell (CART) (HBI0101) for the Treatment of Relapsed and Refractory Multiple Myeloma, International Myeloma Society 20th Annual Meeting. 2023.
NXC-401
CAR-T
CELL
Produced NXC-201, NXC-301, NXC-401
BCMA Surface
Expression
1 NXC-201 BCMA
I Chimeric
Antigen Receptor
I (CAR)
I
I Surface Expression
I.
NXC-301
I Proprietary
Chimeric
I Antigen Receptor
I (CAR)
I
I Surface Expression.
NXC-401 Proprietary
I Chimeric
Antigen Receptor
I (CAR)
●
NXC-201 is a next-generation chimeric antigen receptor (CAR)
T-cell (CAR-T) targeting B-cell maturation antigen (BCMA)
●
●
●●●
IMMİX
S BIOPHARMA
NXC-301 is a next-generation CAR-T with a proprietary target.
Indications:
High Complete Response Rates in AL Amyloidosis and
Multiple Myeloma
●
First Outpatient CAR-T: Short CRS duration (median 1
day) starting on day 0
4% neurotoxicity
Acute lymphocytic leukemia (ALL)
Large B-Cell Lymphoma (LBCL)
Mantle cell lymphoma (MCL)
NXC-401 is a next-generation CAR-T with a proprietary target.
Indications:
Acute Myeloid Leukemia (AML)
61View entire presentation