Neumora Therapeutics IPO Presentation Deck
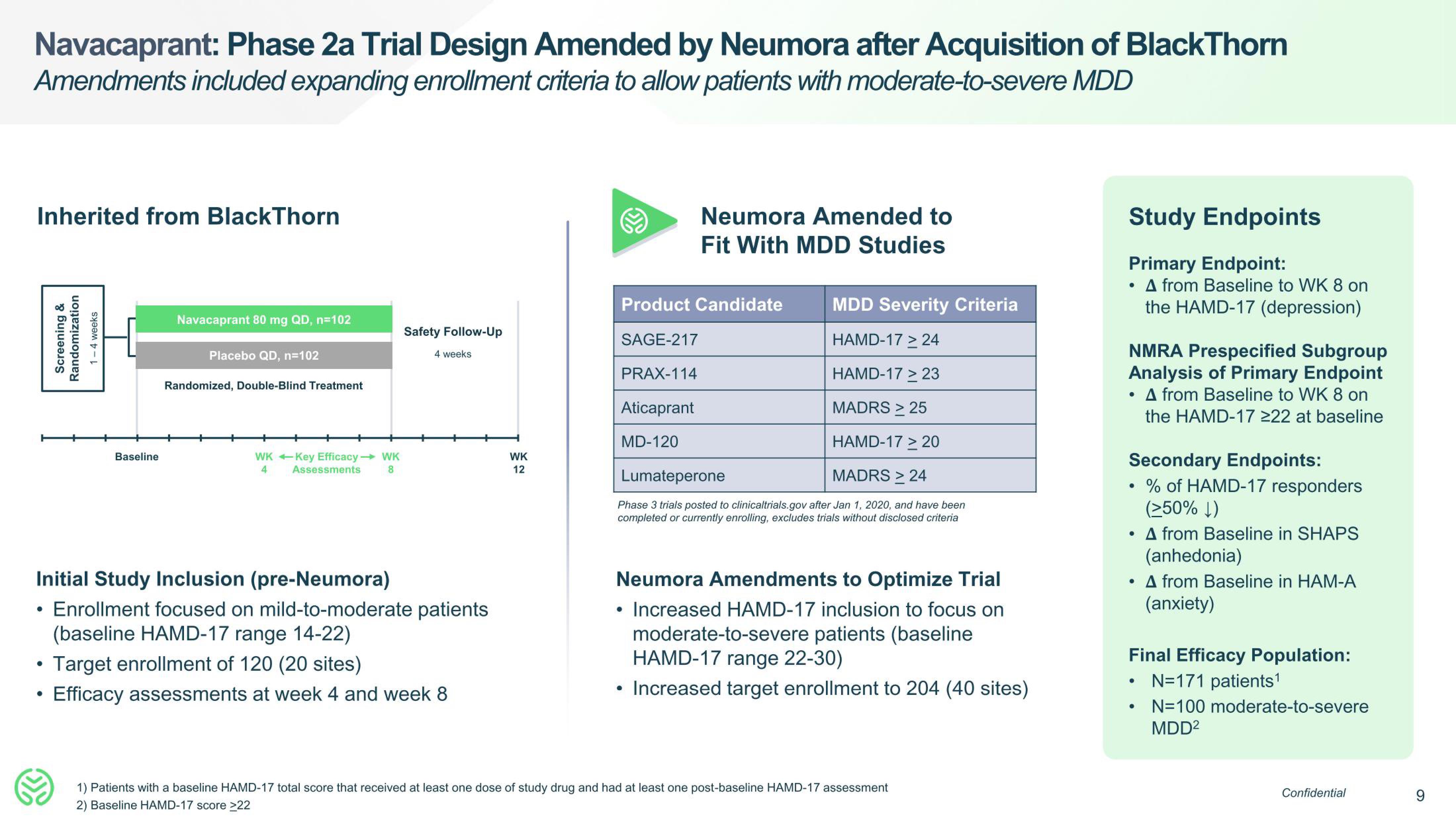
Navacaprant: Phase 2a Trial Design Amended by Neumora after Acquisition of BlackThorn
Amendments included expanding enrollment criteria to allow patients with moderate-to-severe MDD
Inherited from BlackThorn
●
Screening &
Randomization
1-4 weeks
●
Baseline
Navacaprant 80 mg QD, n=102
Placebo QD, n=102
Randomized, Double-Blind Treatment
WK Key Efficacy - WK
8
4
Assessments
Initial Study Inclusion (pre-Neumora)
Enrollment focused on mild-to-moderate patients
(baseline HAMD-17 range 14-22)
Target enrollment of 120 (20 sites)
Efficacy assessments at week 4 and week 8
Safety Follow-Up
4 weeks
WK
12
MDD Severity Criteria
HAMD-17 > 24
HAMD-17 > 23
MADRS > 25
HAMD-17 > 20
Lumateperone
MADRS > 24
Phase 3 trials posted to clinicaltrials.gov after Jan 1, 2020, and have been
completed or currently enrolling, excludes trials without disclosed criteria
Neumora Amended to
Fit With MDD Studies
Product Candidate
SAGE-217
PRAX-114
Aticaprant
MD-120
Neumora Amendments to Optimize Trial
Increased HAMD-17 inclusion to focus on
moderate-to-severe patients (baseline
HAMD-17 range 22-30)
Increased target enrollment to 204 (40 sites)
●
●
1) Patients with a baseline HAMD-17 total score that received at least one dose of study drug and had at least one post-baseline HAMD-17 assessment
2) Baseline HAMD-17 score >22
Study Endpoints
Primary Endpoint:
• A from Baseline to WK 8 on
the HAMD-17 (depression)
NMRA Prespecified Subgroup
Analysis of Primary Endpoint
• A from Baseline to WK 8 on
the HAMD-17 ≥22 at baseline
Secondary Endpoints:
% of HAMD-17 responders
(≥50%)
• A from Baseline in SHAPS
(anhedonia)
• A from Baseline in HAM-A
(anxiety)
Final Efficacy Population:
N=171 patients¹
N=100 moderate-to-severe
MDD²
●
●
Confidential
9View entire presentation