Immix Biopharma Investor Presentation Deck
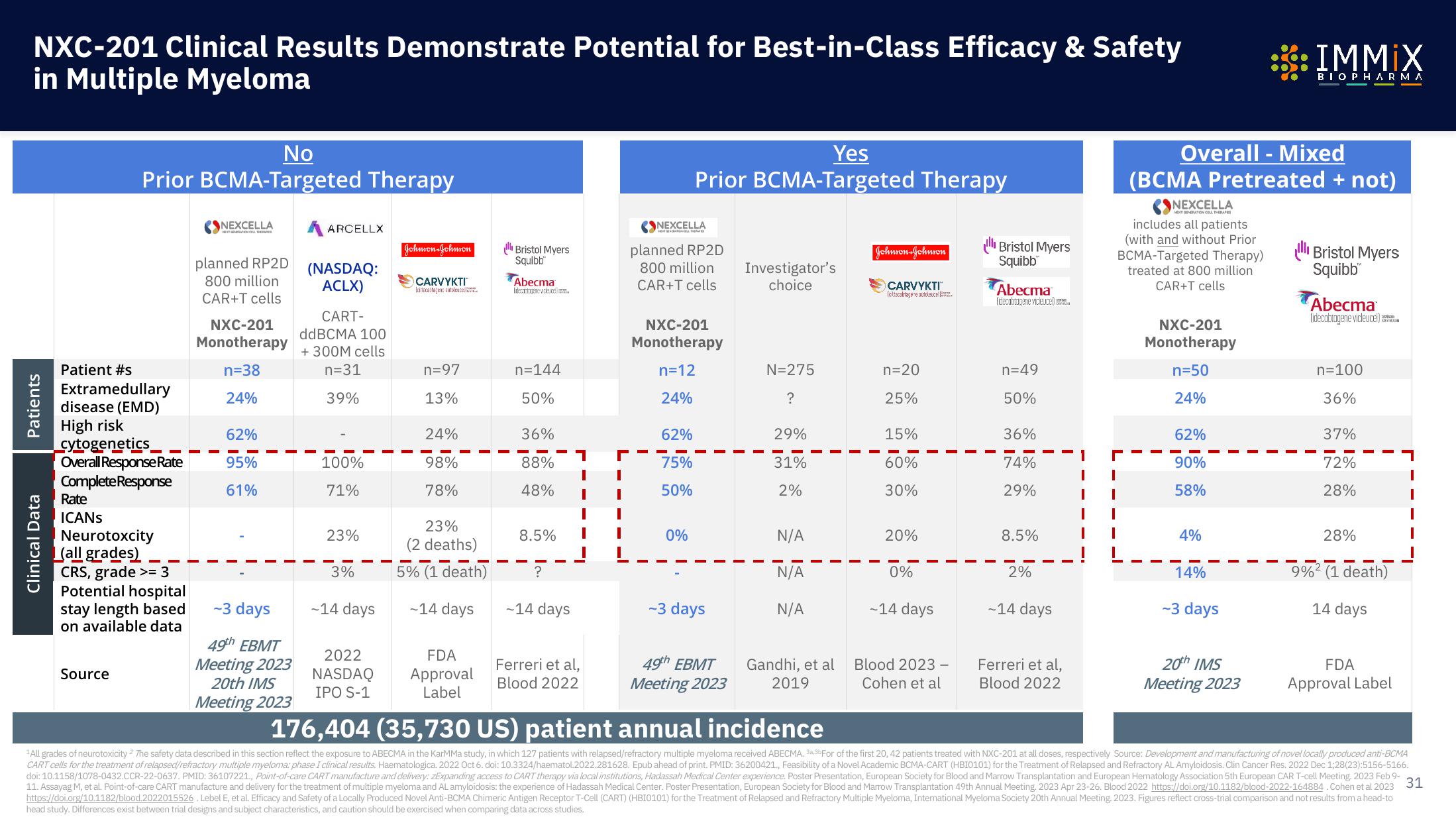
NXC-201 Clinical Results Demonstrate Potential for Best-in-Class Efficacy & Safety
in Multiple Myeloma
Patients
Clinical Data
No
Prior BCMA-Targeted Therapy
Patient #s
Extramedullary
disease (EMD)
High risk
cytogenetics
Overall Response Rate
Complete Response
Rate
ICANS
Neurotoxcity
(all grades)
CRS, grade >= 3
Potential hospital
stay length based
on available data
Source
NEXCELLA
HEXATION CLL THE
planned RP2D
800 million
CAR+T cells
NXC-201
Monotherapy
n=38
24%
62%
95%
61%
-3 days
49th EBMT
Meeting 2023
20th IMS
Meeting 2023
ARCELLX
(NASDAQ:
ACLX)
CART-
ddBCMA 100
+ 300M cells
n=31
39%
100%
71%
23%
3%
-14 days
2022
NASDAQ
IPO S-1
Johnson Johnson
CARVYKTI
fittacattajent autoleucell
n=97
13%
24%
98%
78%
23%
(2 deaths)
5% (1 death)
-14 days
FDA
Approval
Label
Bristol Myers
Squibb
Abecma
lidecatogene videoe
n=144
50%
36%
88%
48%
8.5%
-14 days
Ferreri et al,
Blood 2022
NEXCELLA
Yes
Prior BCMA-Targeted Therapy
planned RP2D
800 million
CAR+T cells
NXC-201
Monotherapy
n=12
24%
62%
75%
50%
0%
-3 days
49th EBMT
Meeting 2023
Investigator's
choice
N=275
?
29%
31%
2%
N/A
N/A
N/A
Gandhi, et al
2019
Johnson Johnson
CARVYKTI
(citacabtagere autoleucel
n=20
25%
15%
60%
30%
20%
0%
-14 days
Blood 2023 -
Cohen et al
Bristol Myers
Squibb
Abecma
(decabtogene vicleuce)
n=49
50%
36%
74%
29%
8.5%
2%
-14 days
Ferreri et al,
Blood 2022
Overall - Mixed
(BCMA Pretreated + not)
NEXCELLA
HET GENERATION CELL THERAP
includes all patients
(with and without Prior
BCMA-Targeted Therapy)
treated at 800 million
CAR+T cells
NXC-201
Monotherapy
n=50
24%
62%
90%
58%
4%
14%
-3 days
●●●
IMMIX
S BIOPHARMA
20th IMS
Meeting 2023
Ill Bristol Myers
Squibb™
Abecma
(idecabtagene vicleuce)
n=100
36%
37%
72%
28%
28%
9%² (1 death)
14 days
FDA
Approval Label
176,404 (35,730 US) patient annual incidence
¹All grades of neurotoxicity 2 The safety data described in this section reflect the exposure to ABECMA in the KarMMa study, in which 127 patients with relapsed/refractory multiple myeloma received ABECMA. 3a3bFor of the first 20, 42 patients treated with NXC-201 at all doses, respectively Source: Development and manufacturing of novel locally produced anti-BCMA
CART cells for the treatment of relapsed/refractory multiple myeloma: phase I clinical results. Haematologica. 2022 Oct 6. doi: 10.3324/haematol.2022.281628. Epub ahead of print. PMID: 36200421, Feasibility of a Novel Academic BCMA-CART (HBI0101) for the Treatment of Relapsed and Refractory AL Amyloidosis. Clin Cancer Res. 2022 Dec 1;28(23):5156-5166.
doi: 10.1158/1078-0432.CCR-22-0637. PMID: 36107221., Point-of-care CART manufacture and delivery: zExpanding access to CART therapy via local institutions, Hadassah Medical Center experience. Poster Presentation, European Society for Blood and Marrow Transplantation and European Hematology Association 5th European CAR T-cell Meeting. 2023 Feb 9-
11. Assayag M, et al. Point-of-care CART manufacture and delivery for the treatment of multiple myeloma and AL amyloidosis: the experience of Hadassah Medical Center. Poster Presentation, European Society for Blood and Marrow Transplantation 49th Annual Meeting. 2023 Apr 23-26. Blood 2022 https://doi.org/10.1182/blood-2022-164884. Cohen et al 2023 31
https://doi.org/10.1182/blood.2022015526. Lebel E, et al. Efficacy and Safety of a Locally Produced Novel Anti-BCMA Chimeric Antigen Receptor T-Cell (CART) (HBI0101) for the Treatment of Relapsed and Refractory Multiple Myeloma, International Myeloma Society 20th Annual Meeting, 2023. Figures reflect cross-trial comparison and not results from a head-to
head study. Differences exist between trial designs and subject characteristics, and caution should be exercised when comparing data across studies.View entire presentation