Genelux Investor Presentation Deck
Durable Benefit of Overall Survival via Clinically-Validated Endpoint
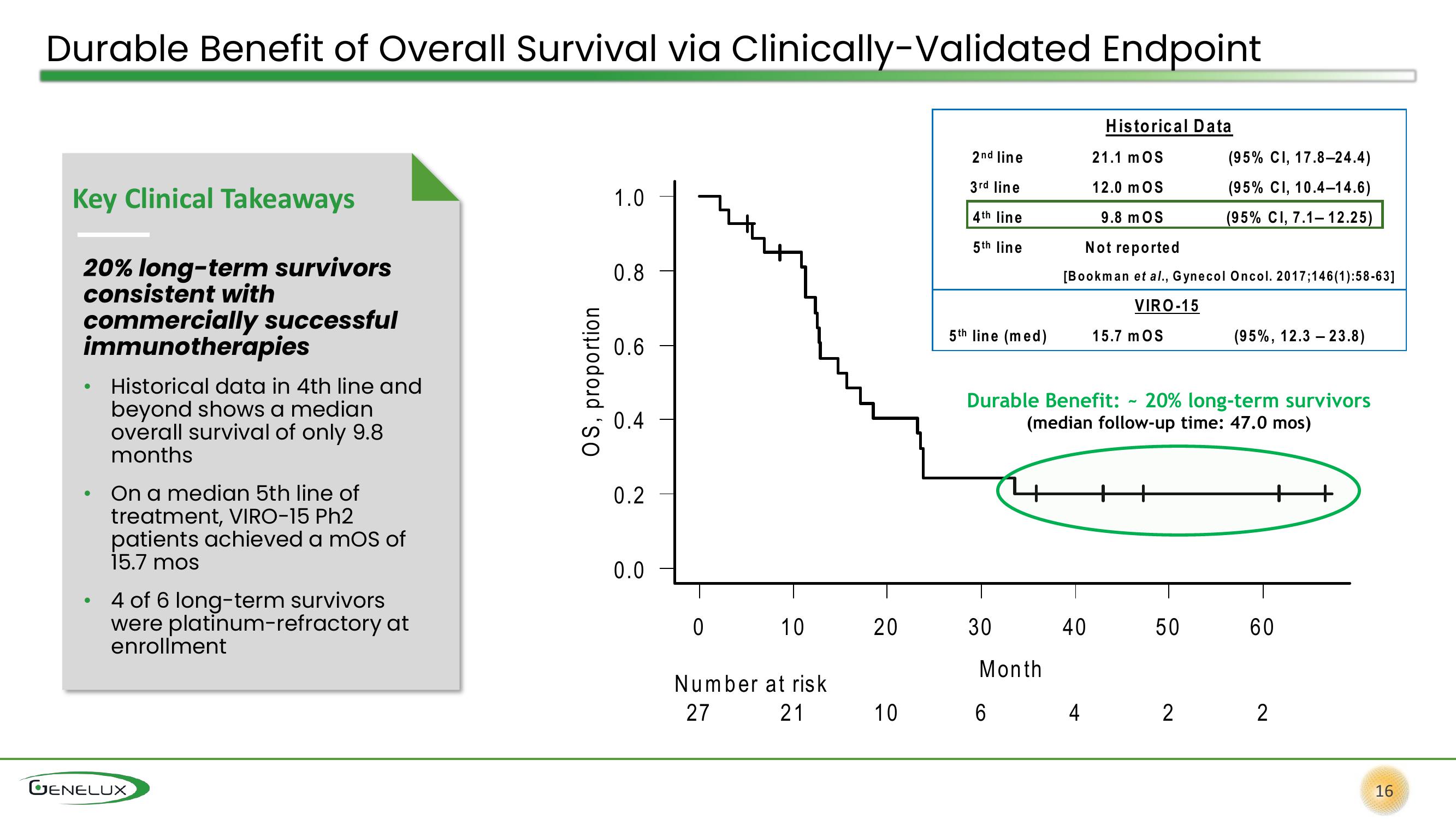
Historical Data
21.1 MOS
12.0 MOS
9.8 MOS
Not reported
[Bookman et al., Gynecol Oncol. 2017;146(1):58-63]
VIRO-15
Key Clinical Takeaways
20% long-term survivors
consistent with
commercially successful
immunotherapies
Historical data in 4th line and
beyond shows a median
overall survival of only 9.8
months
On a median 5th line of
treatment, VIRO-15 Ph2
patients achieved a mos of
15.7 mos
4 of 6 long-term survivors
were platinum-refractory at
enrollment
GENELUX
OS, proportion
1.0
0.8
0.6
0.4
0.2
0.0
0
10
Number at risk
27
21
20
10
2nd line
3rd line
4th line
5th line
5th line (med)
30
Month
Durable Benefit: 20% long-term survivors
(median follow-up time: 47.0 mos)
6
40
15.7 MOS
4
50
(95% CI, 17.8-24.4)
(95% CI, 10.4-14.6)
(95% CI, 7.1-12.25)
2
(95%, 12.323.8)
60
2
16View entire presentation