AstraZeneca Results Presentation Deck
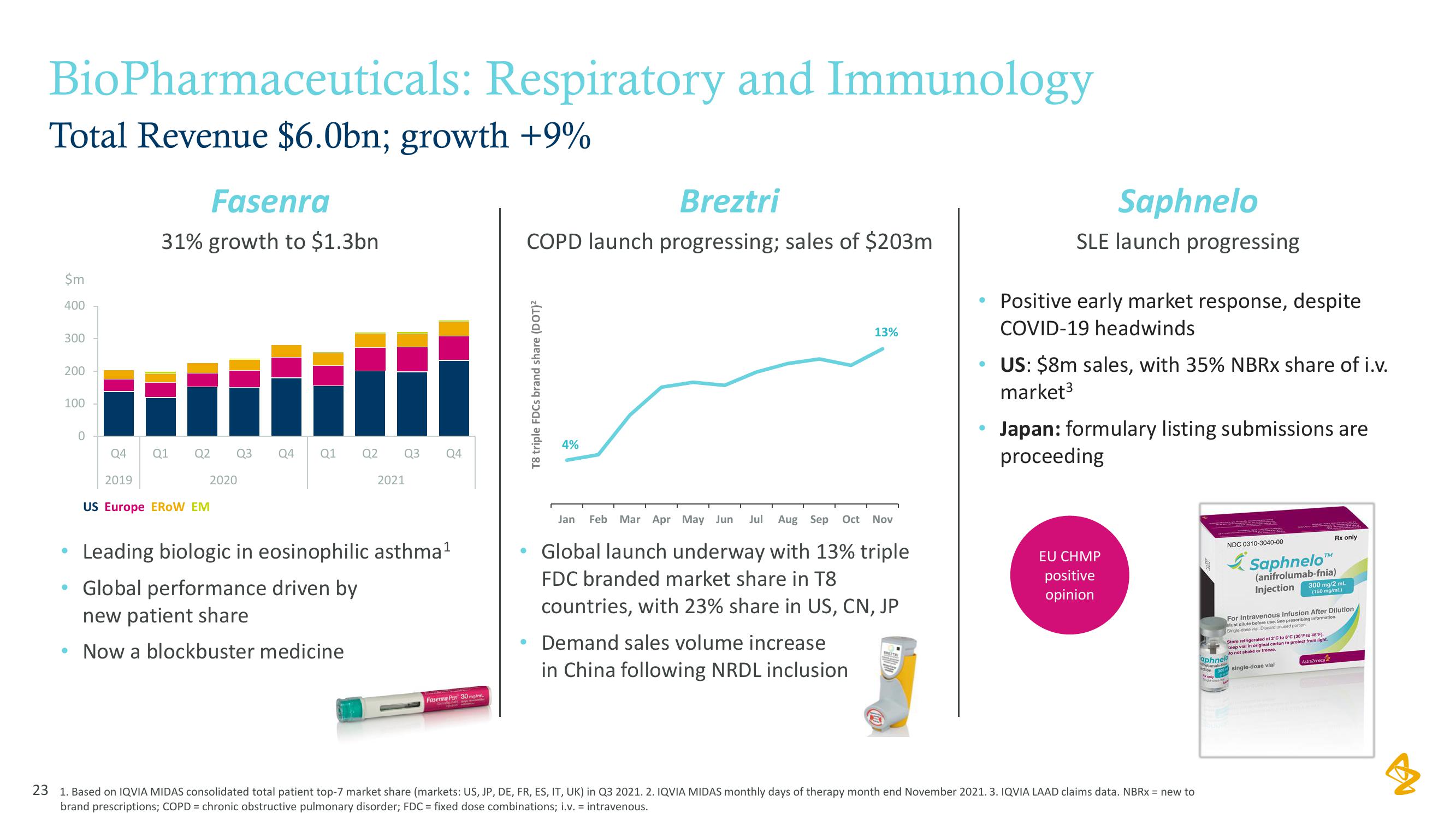
BioPharmaceuticals: Respiratory and Immunology
Total Revenue $6.0bn; growth +9%
$m
400
300
200
100
●
0
●
Q4
2019
Fasenra
31% growth to $1.3bn
Q1 Q2 Q3 Q4 Q1
US Europe EROW EM
2020
Q2
Q3 Q4
Leading biologic in eosinophilic asthma¹
• Global performance driven by
new patient share
Now a blockbuster medicine
2021
Fasen
730 mg/ml
Breztri
COPD launch progressing; sales of $203m
T8 triple FDCs brand share (DOT)²
4%
13%
Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov
Global launch underway with 13% triple
FDC branded market share in T8
countries, with 23% share in US, CN, JP
Demand sales volume increase
in China following NRDL inclusion
Saphnelo
SLE launch progressing
• Positive early market response, despite
COVID-19 headwinds
US: $8m sales, with 35% NBRx share of i.v.
market³
Japan: formulary listing submissions are
proceeding
EU CHMP
positive
opinion
23 1. Based on IQVIA MIDAS consolidated total patient top-7 market share (markets: US, JP, DE, FR, ES, IT, UK) in Q3 2021. 2. IQVIA MIDAS monthly days of therapy month end November 2021. 3. IQVIA LAAD claims data. NBRX = new to
brand prescriptions; COPD = chronic obstructive pulmonary disorder; FDC = fixed dose combinations; i.v. = intravenous.
NDC 0310-3040-00
Saphnelo
(anifrolumab-fnia)
Injection
Rx only
300 mg/2 mL
(150 mg/mL)
For Intravenous Infusion After Dilution
Must dilute before use. See prescribing information.
Single-dose vial Discard unused portion
Store refrigerated at 2°C to 8°C (36°F to 46°F).
Ceep vial in original carton to protect from light.
Jo not shake or freeze.
aphnel
tion 300 single-dose vial
Ma only
AstraZeneca
4View entire presentation