Acquisition of APIRx
For personal use only
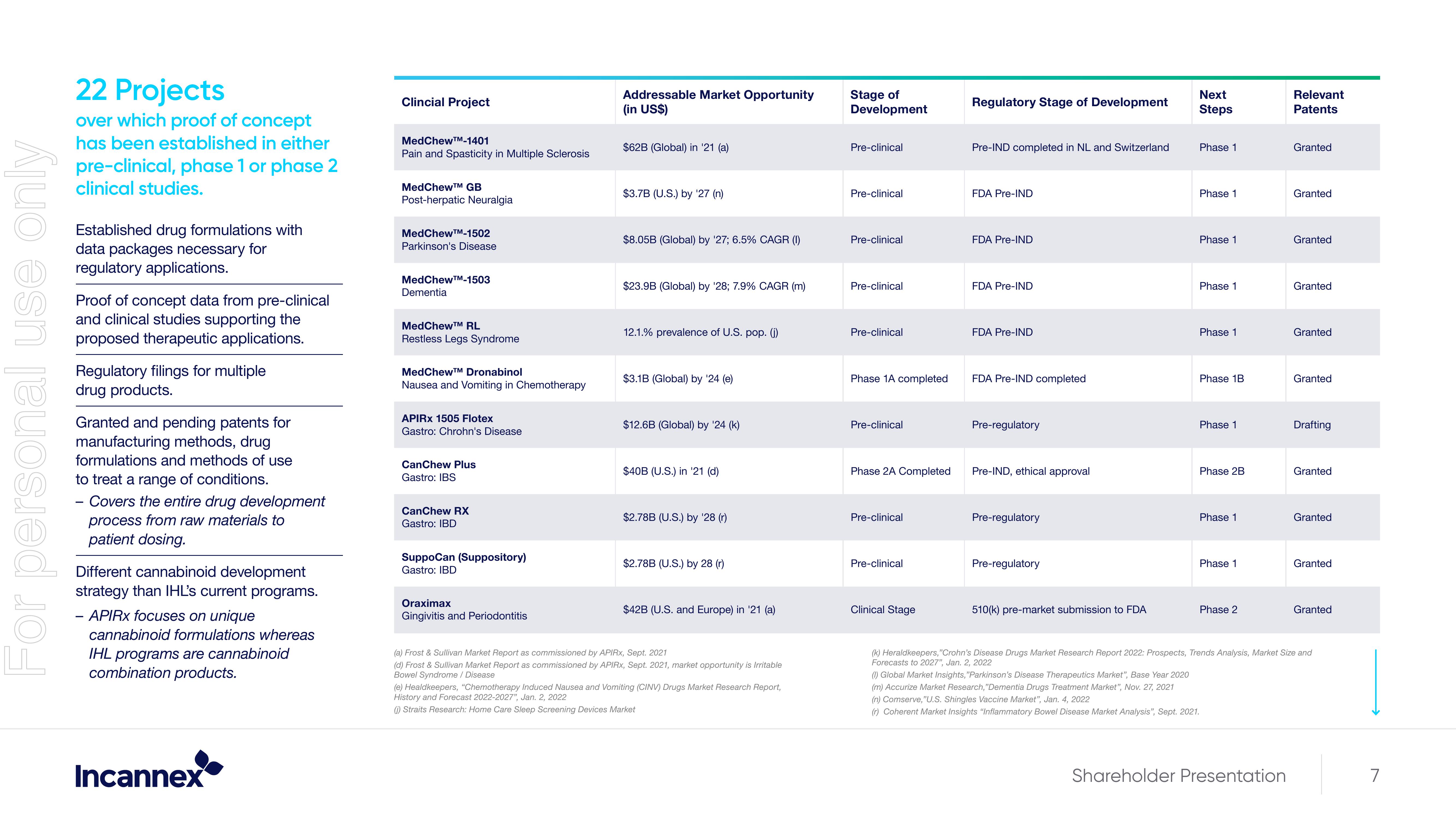
22 Projects
over which proof of concept
has been established in either
pre-clinical, phase 1 or phase 2
clinical studies.
Established drug formulations with
data packages necessary for
regulatory applications.
Proof of concept data from pre-clinical
and clinical studies supporting the
proposed therapeutic applications.
Regulatory filings for multiple
drug products.
Granted and pending patents for
manufacturing methods, drug
formulations and methods of use
to treat a range of conditions.
- Covers the entire drug development
process from raw materials to
patient dosing.
Different cannabinoid development
strategy than IHL's current programs.
- APIRX focuses on unique
cannabinoid formulations whereas
IHL programs are cannabinoid
combination products.
Incannex
Clincial Project
MedChewTM-1401
Pain and Spasticity in Multiple Sclerosis
MedChew™M GB
Post-herpatic Neuralgia
MedChew™-1502
Parkinson's Disease
MedChewTM-1503
Dementia
MedChewTM RL
Restless Legs Syndrome
MedChew™ Dronabinol
Nausea and Vomiting in Chemotherapy
APIRX 1505 Flotex
Gastro: Chrohn's Disease
CanChew Plus
Gastro: IBS
CanChew RX
Gastro: IBD
SuppoCan (Suppository)
Gastro: IBD
Oraximax
Gingivitis and Periodontitis
Addressable Market Opportunity
(in US$)
$62B (Global) in '21 (a)
$3.7B (U.S.) by '27 (n)
$8.05B (Global) by '27; 6.5% CAGR (1)
$23.9B (Global) by '28; 7.9% CAGR (m)
12.1.% prevalence of U.S. pop. (j)
$3.1B (Global) by '24 (e)
$12.6B (Global) by '24 (k)
$40B (U.S.) in '21 (d)
$2.78B (U.S.) by '28 (r)
$2.78B (U.S.) by 28 (r)
$42B (U.S. and Europe) in '21 (a)
(a) Frost & Sullivan Market Report as commissioned by APIRX, Sept. 2021
(d) Frost & Sullivan Market Report as commissioned by APIRX, Sept. 2021, market opportunity is Irritable
Bowel Syndrome / Disease
(e) Healdkeepers, "Chemotherapy Induced Nausea and Vomiting (CINV) Drugs Market Research Report,
History and Forecast 2022-2027", Jan. 2, 2022
() Straits Research: Home Care Sleep Screening Devices Market
Stage of
Development
Pre-clinical
Pre-clinical
Pre-clinical
Pre-clinical
Pre-clinical
Phase 1A completed
Pre-clinical
Phase 2A Completed
Pre-clinical
Pre-clinical
Clinical Stage
Regulatory Stage of Development
Pre-IND completed in NL and Switzerland
FDA Pre-IND
FDA Pre-IND
FDA Pre-IND
FDA Pre-IND
FDA Pre-IND completed
Pre-regulatory
Pre-IND, ethical approval
Pre-regulatory
Pre-regulatory
510(k) pre-market submission to FDA
Next
Steps
(1) Global Market Insights, "Parkinson's Disease Therapeutics Market", Base Year 2020
(m) Accurize Market Research, "Dementia Drugs Treatment Market", Nov. 27, 2021
Phase 1
Phase 1
Phase 1
Phase 1
Phase 1
Phase 1B
Phase 1
Phase 2B
Phase 1
Phase 1
Phase 2
(n) Comserve,"U.S. Shingles Vaccine Market", Jan. 4, 2022
(r) Coherent Market Insights "Inflammatory Bowel Disease Market Analysis", Sept. 2021.
Relevant
Patents
Shareholder Presentation
Granted
Granted
Granted
Granted
Granted
Granted
Drafting
Granted
Granted
(k) Heraldkeepers, "Crohn's Disease Drugs Market Research Report 2022: Prospects, Trends Analysis, Market Size and
Forecasts to 2027", Jan. 2, 2022
Granted
Granted
7View entire presentation