IDEAYA Biosciences Interim IDE397 Phase 1 Clinical Data and Q1 2022 Corporate Update
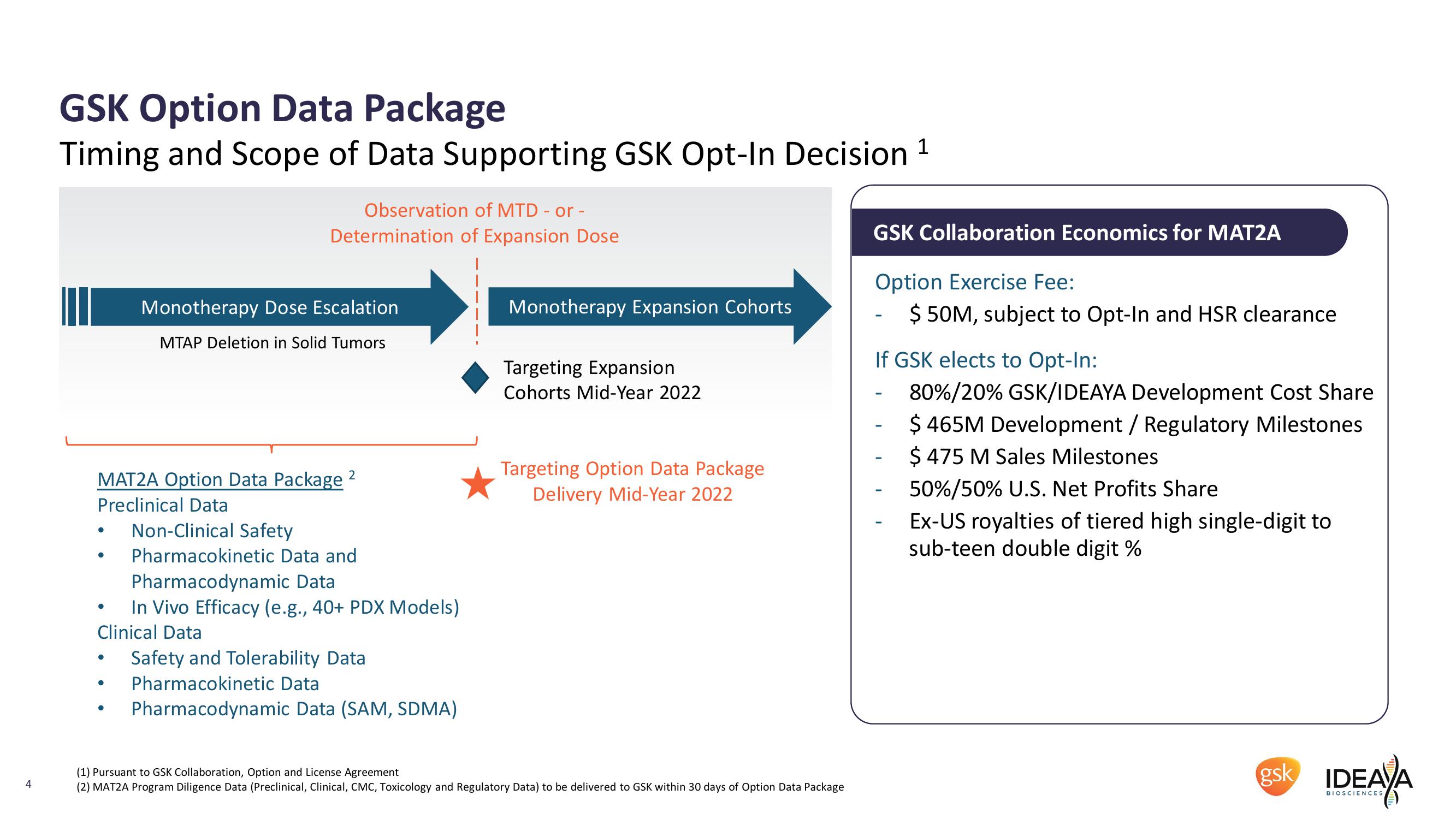
GSK Option Data Package
Timing and Scope of Data Supporting GSK Opt-In Decision 1
Observation of MTD - or -
Determination of Expansion Dose
Monotherapy Dose Escalation
MTAP Deletion in Solid Tumors
Monotherapy Expansion Cohorts
Targeting Expansion
Cohorts Mid-Year 2022
MAT2A Option Data Package
2
Targeting Option Data Package
Delivery Mid-Year 2022
Preclinical Data
Non-Clinical Safety
GSK Collaboration Economics for MAT2A
Option Exercise Fee:
$50M, subject to Opt-In and HSR clearance
If GSK elects to Opt-In:
80%/20% GSK/IDEAYA Development Cost Share
$ 465M Development / Regulatory Milestones
$ 475 M Sales Milestones
50%/50% U.S. Net Profits Share
Ex-US royalties of tiered high single-digit to
sub-teen double digit %
Pharmacokinetic Data and
Pharmacodynamic Data
In Vivo Efficacy (e.g., 40+ PDX Models)
Clinical Data
•
.
Safety and Tolerability Data
Pharmacokinetic Data
Pharmacodynamic Data (SAM, SDMA)
(1) Pursuant to GSK Collaboration, Option and License Agreement
4
(2) MAT2A Program Diligence Data (Preclinical, Clinical, CMC, Toxicology and Regulatory Data) to be delivered to GSK within 30 days of Option Data Package
gsk
IDEAYA
BIOSCIENCESView entire presentation