Imara M&A
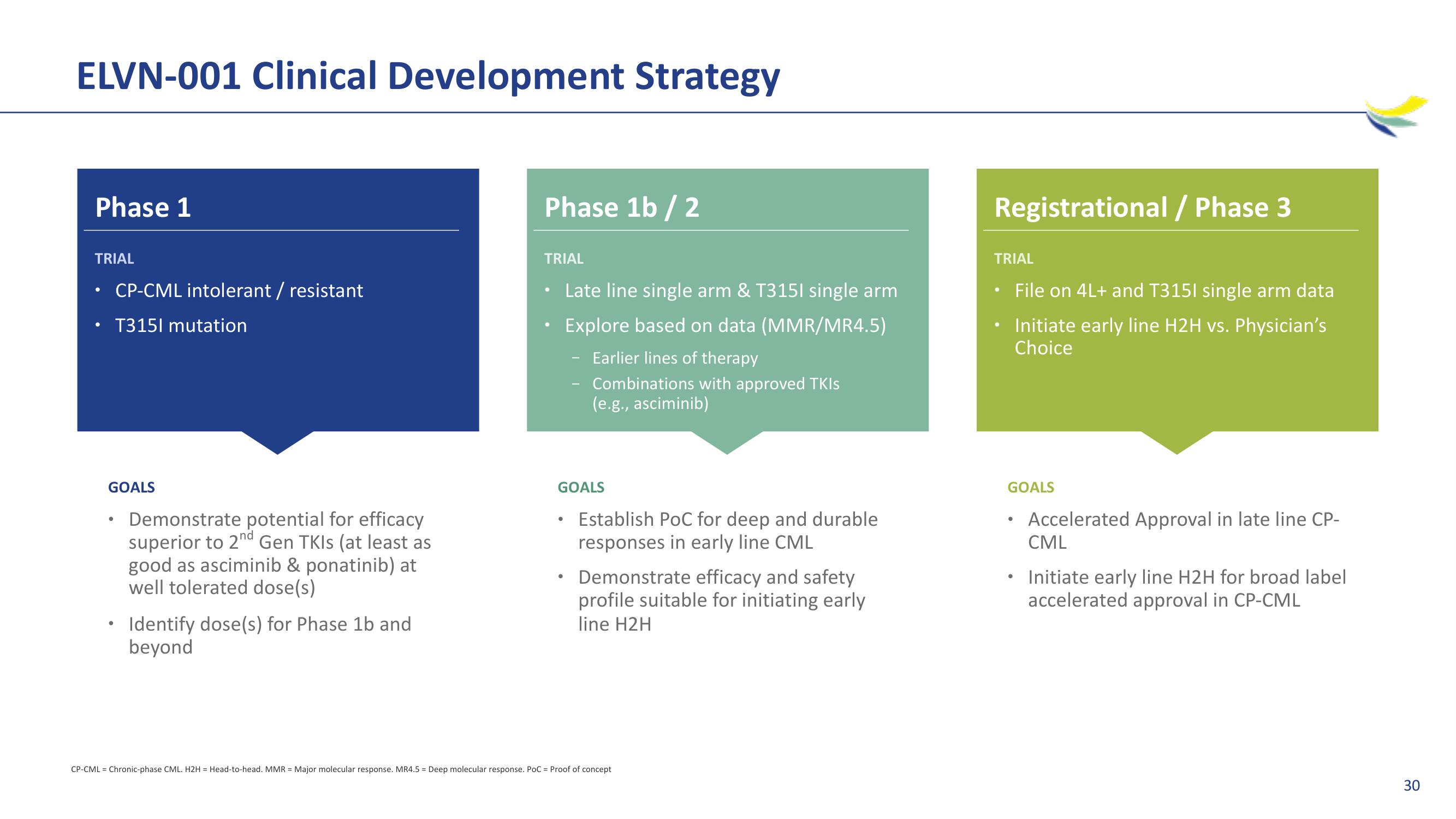
ELVN-001 Clinical Development Strategy
Phase 1
TRIAL
●
●
CP-CML intolerant / resistant
T3151 mutation
GOALS
Demonstrate potential for efficacy
superior to 2nd Gen TKIs (at least as
good as asciminib & ponatinib) at
well tolerated dose(s)
Identify dose(s) for Phase 1b and
beyond
Phase 1b / 2
TRIAL
GOALS
●
Late line single arm & T3151 single arm
Explore based on data (MMR/MR4.5)
Earlier lines of therapy
Combinations with approved TKIs
(e.g., asciminib)
●
Establish PoC for deep and durable
responses in early line CML
Demonstrate efficacy and safety
profile suitable for initiating early
line H2H
CP-CML = Chronic-phase CML. H2H = Head-to-head. MMR = Major molecular response. MR4.5 = Deep molecular response. PoC = Proof of concept
Registrational / Phase 3
TRIAL
GOALS
●
File on 4L+ and T3151 single arm data
Initiate early line H2H vs. Physician's
Choice
●
Accelerated Approval in late line CP-
CML
Initiate early line H2H for broad label
accelerated approval in CP-CML
30View entire presentation