BioNTech Investor Day Presentation Deck
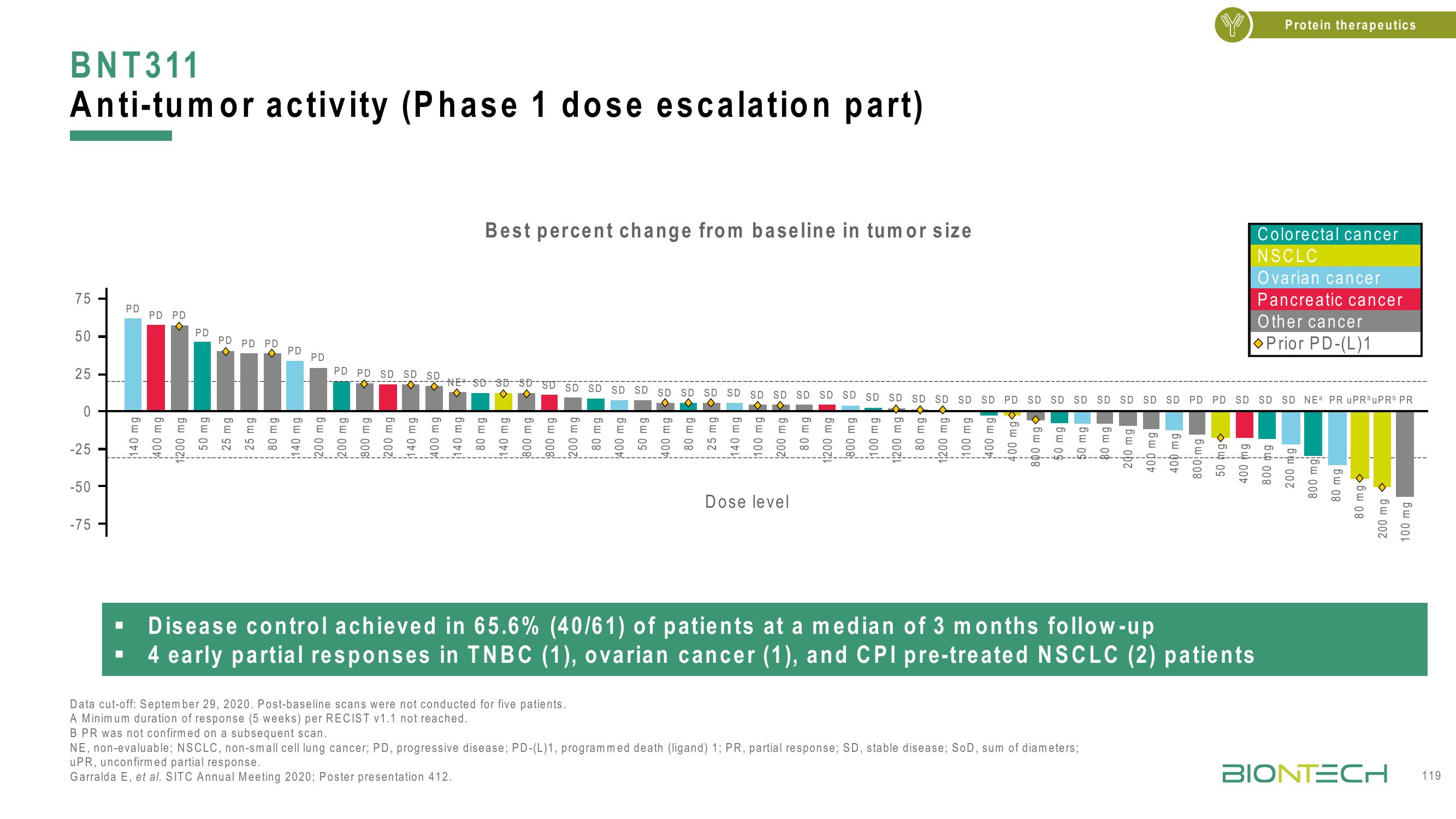
BNT311
Anti-tumor activity (Phase 1 dose escalation part)
75
50
25
0
-25
-50
-75
IL
T
1
PD
PD PD
PD
PD PD PD
mg
mg
mg
400 mg
11200 mg
50 mg
25 mg
25
140
80
PD
140 mg
PD
200 mg
PD PD SD SD SD
200 mg
1800 mg
200 mg
500 N N
Best percent change from baseline in tumor size
140 mg
400 mg
140 mg
80 mg
140 mg
800 mg
800 mg
200 mg
80 mg
400 mg
50 mg
400 mg
80 mg
25 mg
140 mg
100 mg
200 mg
80 mg
1200 mg
800 mg
100 mg
1200 mg
80 mg
1200 mg
100 mg
400 mg
-NE-SD-SD--SD-SD SD SD SD SD SD SD SD SD SD SD SD SD SD SD SD SD SD SD SD PD SD SD SD SD SD SD SD PD PD SD SD SD NEa PR uPRbuPRD PR
Dose level
Data cut-off: September 29, 2020. Post-baseline scans were not conducted for five patients.
A Minimum duration of response (5 weeks) per RECIST v1.1 not reached.
B PR was not confirmed on a subsequent scan.
400 mg
800 mg
50 mg
50 mg
80 mg
200 mg
400 mg
400 mg
Disease
control
achieved in 65.6%
(40/61) of patients at a median of 3 months follow-up
4 early partial responses in TNBC (1), ovarian cancer (1), and CPI pre-treated NSCLC (2) patients
Protein therapeutics
NE, non-evaluable; NSCLC, non-small cell lung cancer; PD, progressive disease; PD-(L)1, programmed death (ligand) 1; PR, partial response; SD, stable disease; SoD, sum of diameters;
uPR, unconfirmed partial response.
Garralda E, et al. SITC Annual Meeting 2020; Poster presentation 412.
Colorectal cancer
NSCLC
Ovarian cancer
Pancreatic cancer
Other cancer
Prior PD-(L)1
800 mg
50 mg
400 mg
800 mg
200 mg
800 mg
80 mg
80 mg
200 mg
100 mg
BIONTECH
119View entire presentation