BioNTech Results Presentation Deck
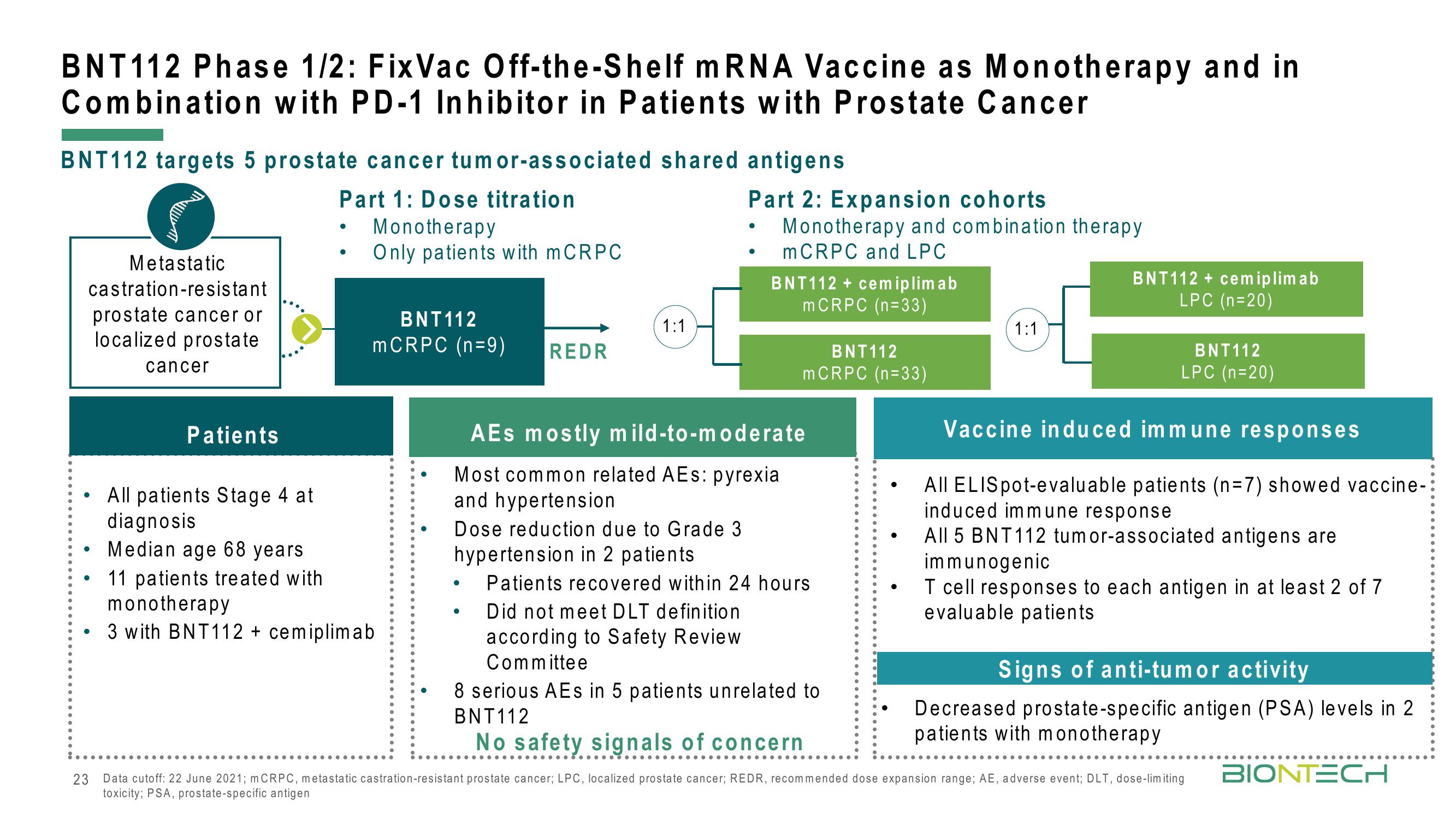
BNT112 Phase 1/2: Fix Vac Off-the-Shelf mRNA Vaccine as Monotherapy and in
Combination with PD-1 Inhibitor in Patients with Prostate Cancer
BNT112 targets 5 prostate cancer tumor-associated shared antigens
Part 1: Dose titration
Monotherapy
Only patients with mCRPC
Metastatic
castration-resistant
●
●
23
prostate cancer or
localized prostate
cancer
Patients
All patients Stage 4 at
diagnosis
Median age 68 years
●
●
BNT112
mCRPC (n=9)
11 patients treated with
monotherapy
3 with BNT112 + cemiplimab
REDR
1:1
Part 2: Expansion cohorts
Monotherapy and combination therapy
mCRPC and LPC
●
Did not meet DLT definition
according to Safety Review.
Committee
BNT112 + cemiplimab
mCRPC (n=33)
BNT112
mCRPC (n=33)
AEs mostly mild-to-moderate
Most common related AEs: pyrexia
and hypertension
Dose reduction due to Grade 3
hypertension in 2 patients.
Patients recovered within 24 hours
8 serious AEs in 5 patients unrelated to
BNT112
No safety signals of concern
1:1
BNT112 + cemiplimab
LPC (n=20)
BNT112
LPC (n=20)
Vaccine induced immune responses
All ELISpot-evaluable patients (n=7) showed vaccine-
induced immune response
All 5 BNT112 tumor-associated antigens are
immunogenic
T cell responses to each antigen in at least 2 of 7
evaluable patients
Signs of anti-tumor activity
Decreased prostate-specific antigen (PSA) levels in 2
patients with monotherapy
Data cutoff: 22 June 2021; mCRPC, metastatic castration-resistant prostate cancer; LPC, localized prostate cancer; REDR, recommended dose expansion range; AE, adverse event; DLT, dose-limiting
toxicity; PSA, prostate-specific antigen
.......
BIONTECHView entire presentation