Ocuphire Pharma Results Presentation Deck
27
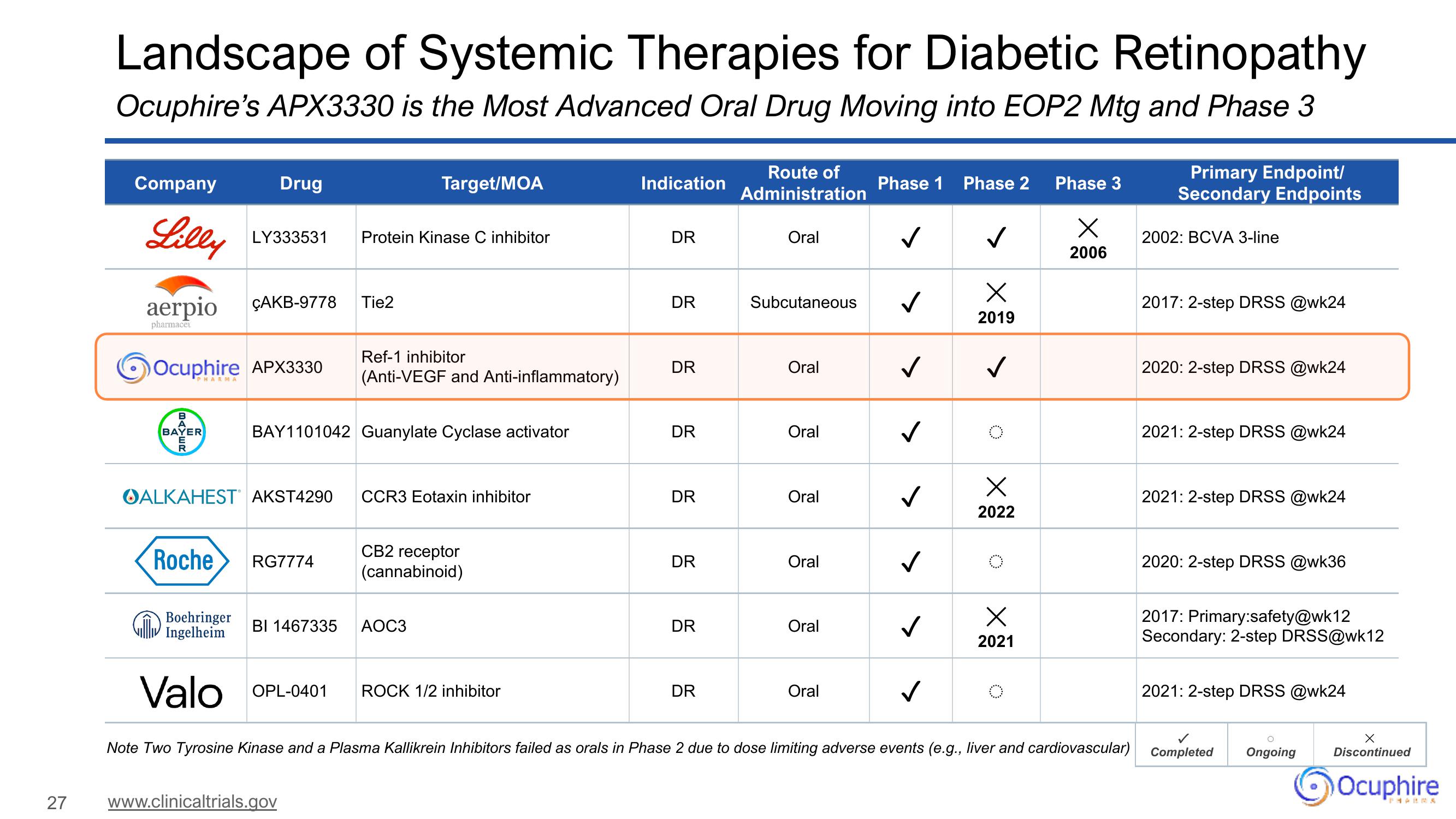
Landscape of Systemic Therapies for Diabetic Retinopathy
Ocuphire's APX3330 is the Most Advanced Oral Drug Moving into EOP2 Mtg and Phase 3
Company
Lilly
aerpio
pharmacei
B
BAYER
E
R
Drug
LY333531
ÇAKB-9778
Ocuphire APX3330
OALKAHESTⓇ AKST4290
Roche RG7774
Boehringer
Ingelheim
Valo OPL-0401
BAY1101042 Guanylate Cyclase activator
Protein Kinase C inhibitor
Tie2
www.clinicaltrials.gov
Target/MOA
Ref-1 inhibitor
(Anti-VEGF and Anti-inflammatory)
CCR3 Eotaxin inhibitor
BI 1467335 AOC3
CB2 receptor
(cannabinoid)
ROCK 1/2 inhibitor
Indication
DR
DR
DR
DR
DR
DR
DR
DR
Route of
Administration
Oral
Subcutaneous
Oral
Oral
Oral
Oral
Oral
Oral
Phase 1 Phase 2 Phase 3
X
2006
✓
✓
✓
✓
✓
✓
✓
✓
✓
X
2019
✓
X
2022
X
2021
Note Two Tyrosine Kinase and a Plasma Kallikrein Inhibitors failed as orals in Phase 2 due to dose limiting adverse events (e.g., liver and cardiovascular)
Primary Endpoint/
Secondary Endpoints
2002: BCVA 3-line
2017: 2-step DRSS @wk24
2020: 2-step DRSS @wk24
2021: 2-step DRSS @wk24
2021: 2-step DRSS @wk24
2020: 2-step DRSS @wk36
2017: Primary:safety@wk12
Secondary: 2-step DRSS@wk12
2021: 2-step DRSS @wk24
Completed Ongoing
X
Discontinued
Ocuphire
FHPERSView entire presentation