BioNTech Results Presentation Deck
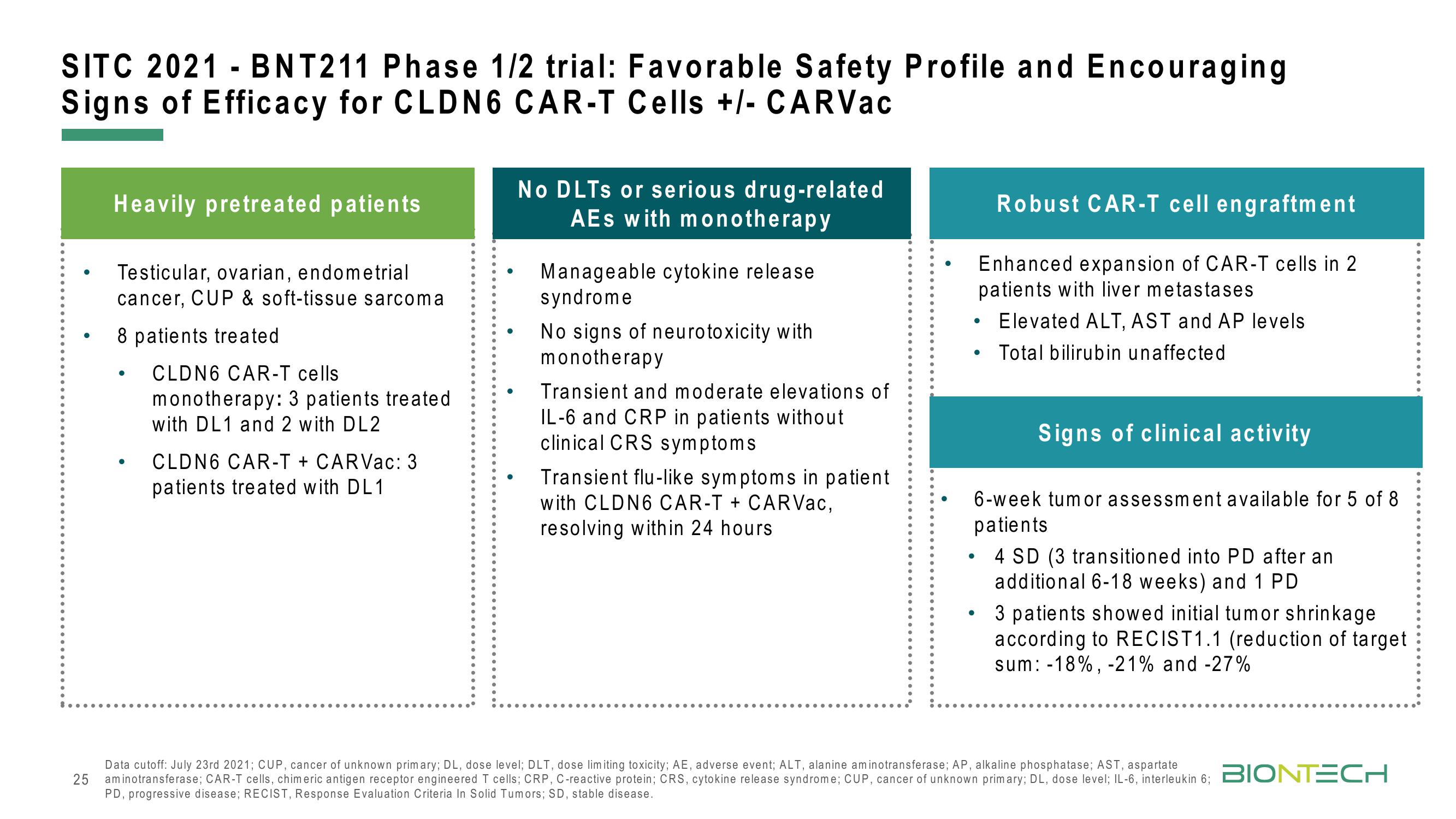
SITC 2021 - BNT211 Phase 1/2 trial: Favorable Safety Profile and Encouraging
Signs of Efficacy for CLDN6 CAR-T Cells +/- CARVac
●
●
25
Heavily pretreated patients
Testicular, ovarian, endometrial
cancer, CUP & soft-tissue sarcoma
8 patients treated
●
CLDN6 CAR-T cells
monotherapy: 3 patients treated.
with DL1 and 2 with DL2
CLDN6 CAR-T + CARVac: 3
patients treated with DL1
No DLTs or serious drug-related
AEs with monotherapy
Manageable cytokine release.
syndrome
No signs of neurotoxicity with
monotherapy
Transient and moderate elevations of
IL-6 and CRP in patients without
clinical CRS symptoms
Transient flu-like symptoms in patient
with CLDN6 CAR-T + CARVac,
resolving within 24 hours
Robust CAR-T cell engraftment
Enhanced expansion of CAR-T cells in 2
patients with liver metastases
Elevated ALT, AST and AP levels
• Total bilirubin unaffected
●
Signs of clinical activity
6-week tumor assessment available for 5 of 8
patients
●
4 SD (3 transitioned into PD after an
additional 6-18 weeks) and 1 PD
• 3 patients showed initial tumor shrinkage
according to RECIST1.1 (reduction of target
sum: -18%, -21% and -27%
Data cutoff: July 23rd 2021; CUP, cancer of unknown primary; DL, dose level; DLT, dose toxicity; AE, adverse event; ALT, alanine AP, alkaline phosphatase; AST,
aminotransferase; CAR-T cells, chimeric antigen receptor engineered T cells; CRP, C-reactive protein; CRS, cytokine release syndrome; CUP, cancer of unknown primary; DL, dose level; IL-6, interleukin 6; BIONTECH
PD, progressive disease; RECIST, Response Evaluation Criteria In Solid Tumors; SD, stable disease.View entire presentation