Imara M&A
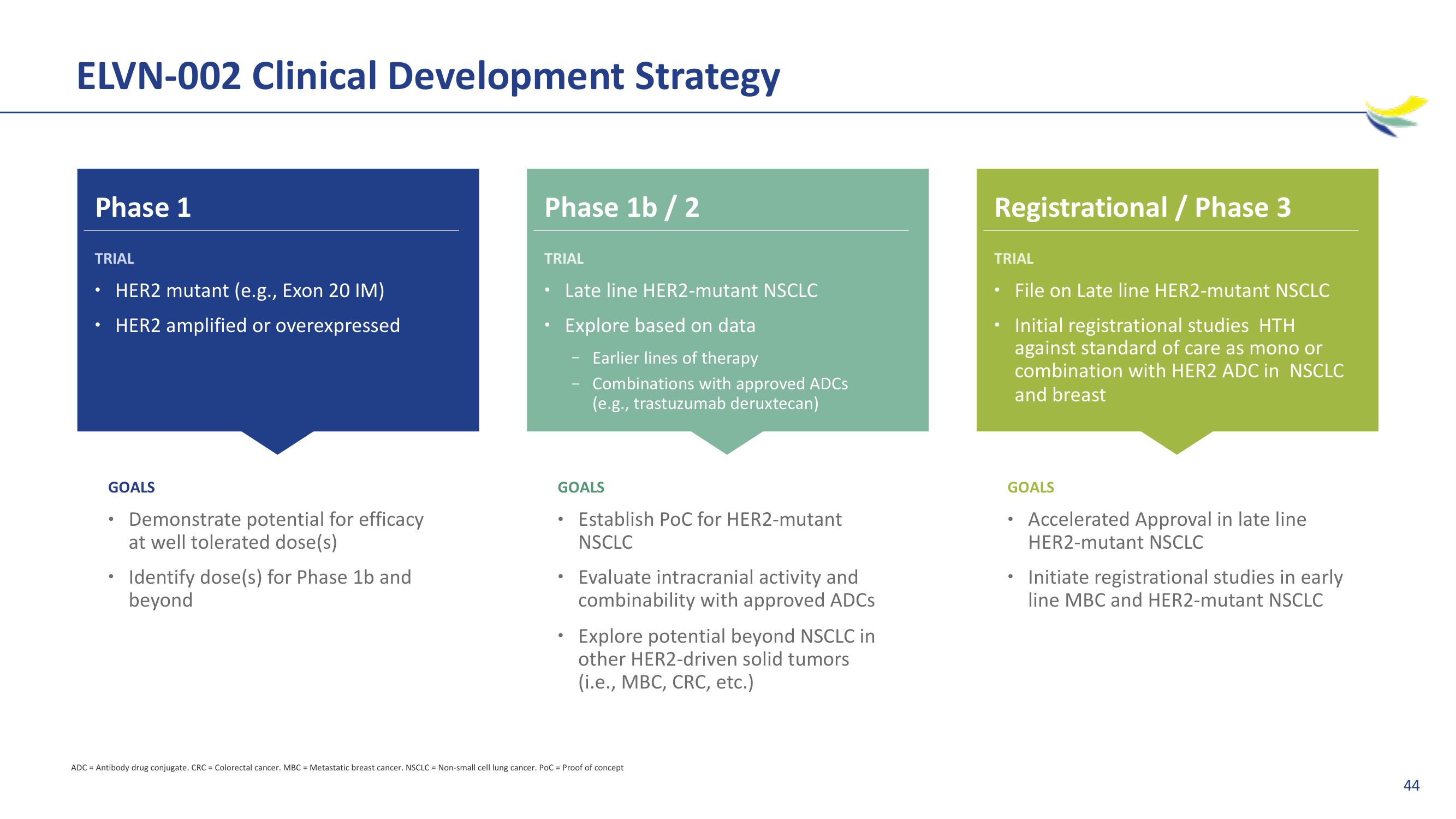
ELVN-002 Clinical Development Strategy
Phase 1
TRIAL
●
HER2 mutant (e.g., Exon 20 IM)
HER2 amplified or overexpressed
GOALS
●
Demonstrate potential for efficacy
at well tolerated dose(s)
Identify dose(s) for Phase 1b and
beyond
Phase 1b / 2
TRIAL
●
Late line HER2-mutant NSCLC
Explore based on data
GOALS
●
Earlier lines of therapy
Combinations with approved ADCs
(e.g., trastuzumab deruxtecan)
Establish PoC for HER2-mutant
NSCLC
Evaluate intracranial activity and
combinability with approved ADCs
Explore potential beyond NSCLC in
other HER2-driven solid tumors
(i.e., MBC, CRC, etc.)
ADC = Antibody drug conjugate. CRC = Colorectal cancer. MBC = Metastatic breast cancer. NSCLC = Non-small cell lung cancer. PoC = Proof of concept
Registrational / Phase 3
TRIAL
GOALS
●
File on Late line HER2-mutant NSCLC
Initial registrational studies HTH
against standard of care as mono or
combination with HER2 ADC in NSCLC
and breast
●
Accelerated Approval in late line
HER2-mutant NSCLC
Initiate registrational studies in early
line MBC and HER2-mutant NSCLC
44View entire presentation